Benzaldehyde and CH3COCl can be distinguished by
A
Tollen's test
B
Bendict's test
C
Iodoform test
D
2,4,DNP test
Text Solution
The correct Answer is:B, C
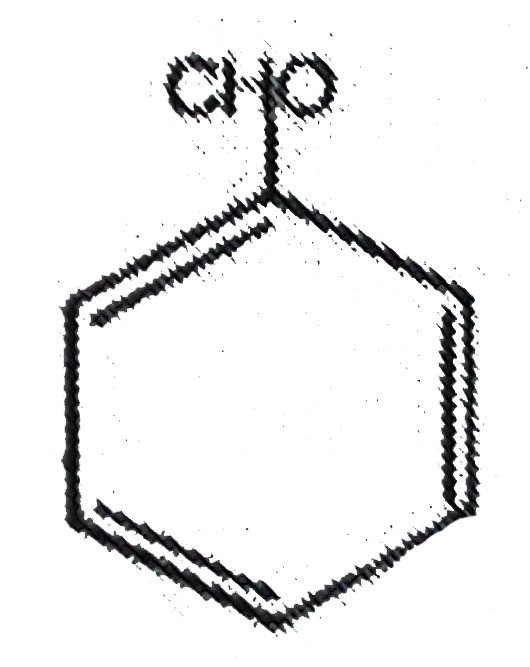
gives Tollen's test 2,4-DNP test, Benedict's test, iodoform test and does not give Fehling's test. CH3CHO gives Tollen's test 2,4DNP test, Fehling's test, Benedict's test iodoform test . Hence choices (b) and (c) are correct but choice (a) and (d) are incorrect.
|
Similar Questions
Knowledge Check
HCOOH and CH3COOH can be distinguished by
ATollen's reagentBFehling's solutionCKMnO4DNaHCO3HCOOH and CH3COOH can be distinguished by
ATollen's reagentBFehling's solutionCKKnO4DNaHCO3Acetic acid and CH3COCl can be distinguished by
ANaHCO3 testBNa metal testCEster formation estDBr2 (aq) test
Benzaldehyde and acetaldehyde can be distinguish by :
View SolutionBenzaldehyde and acetone can be best distinguished using ……..
View SolutionBenzaldehyde and acetone can be best distinguished using ……..
View SolutionBenzaldehyde and acetone can be best distinguished using ……..
View SolutionAcetaldehyde and benzaldehyde can not be distinguished by
View Solution

