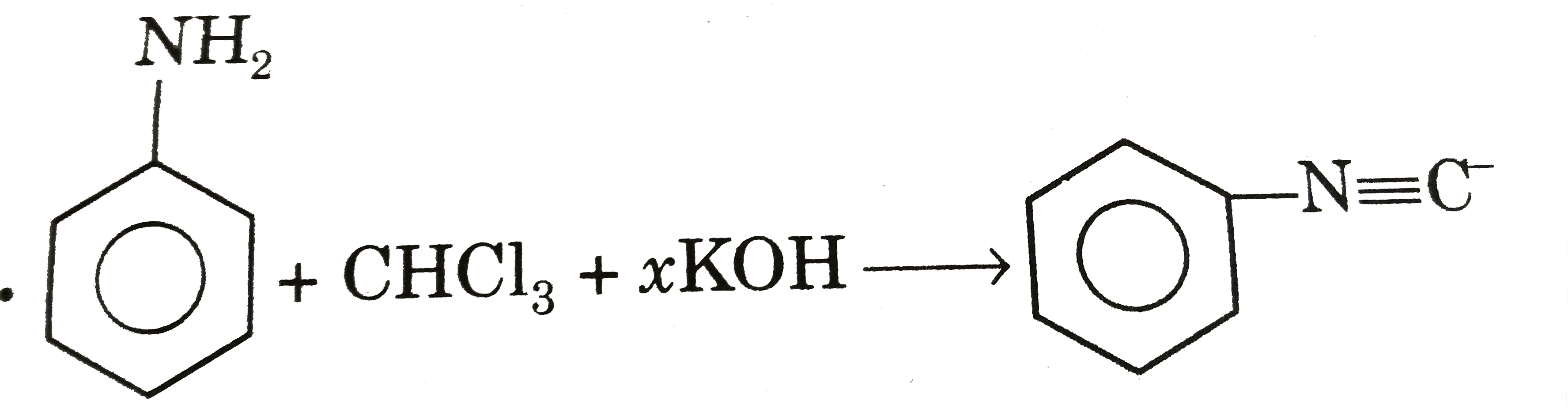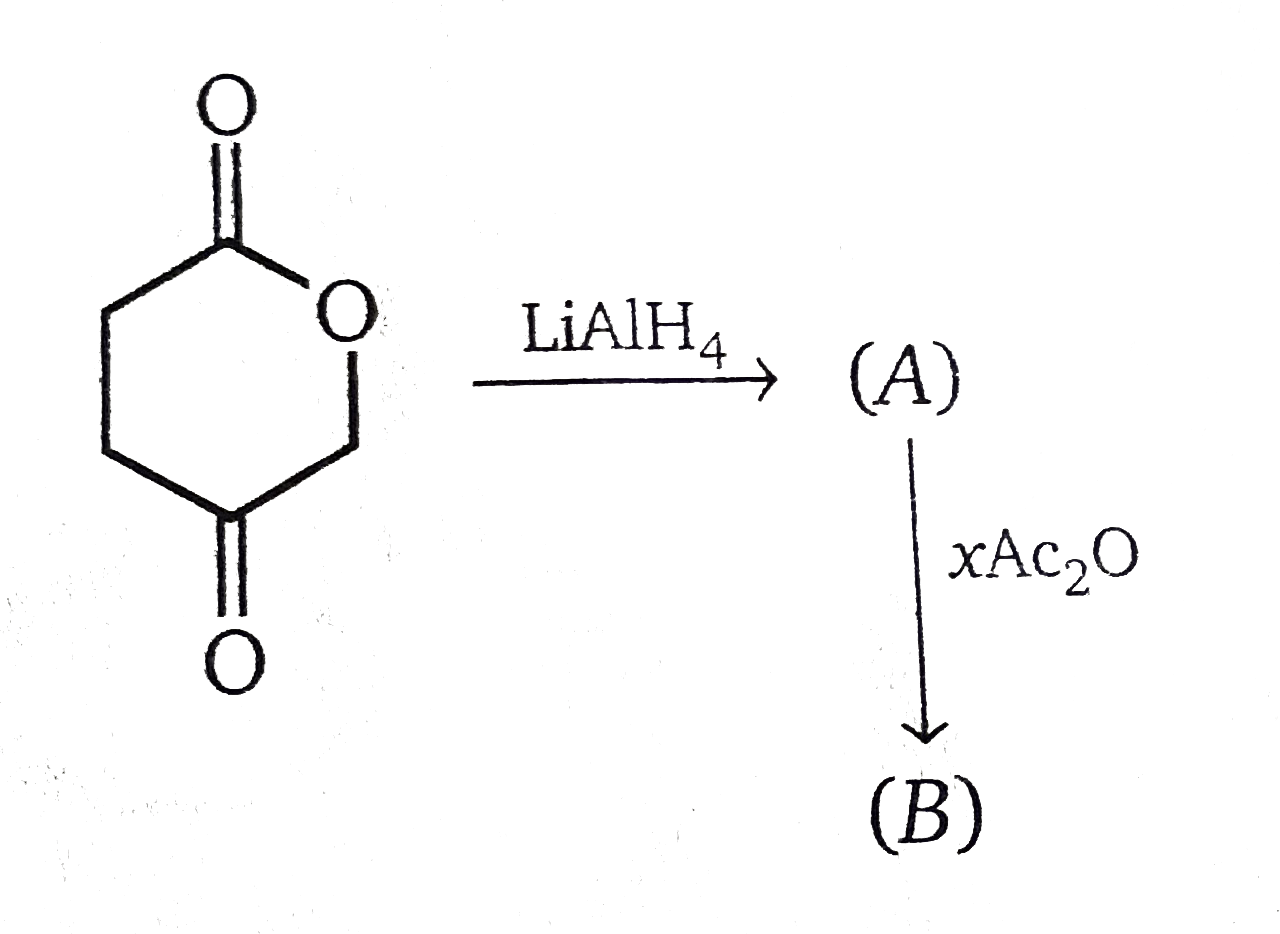
x= moles of anhydride consumed
A
1
B
2
C
3
D
4
Video Solution
Text Solution
The correct Answer is:C
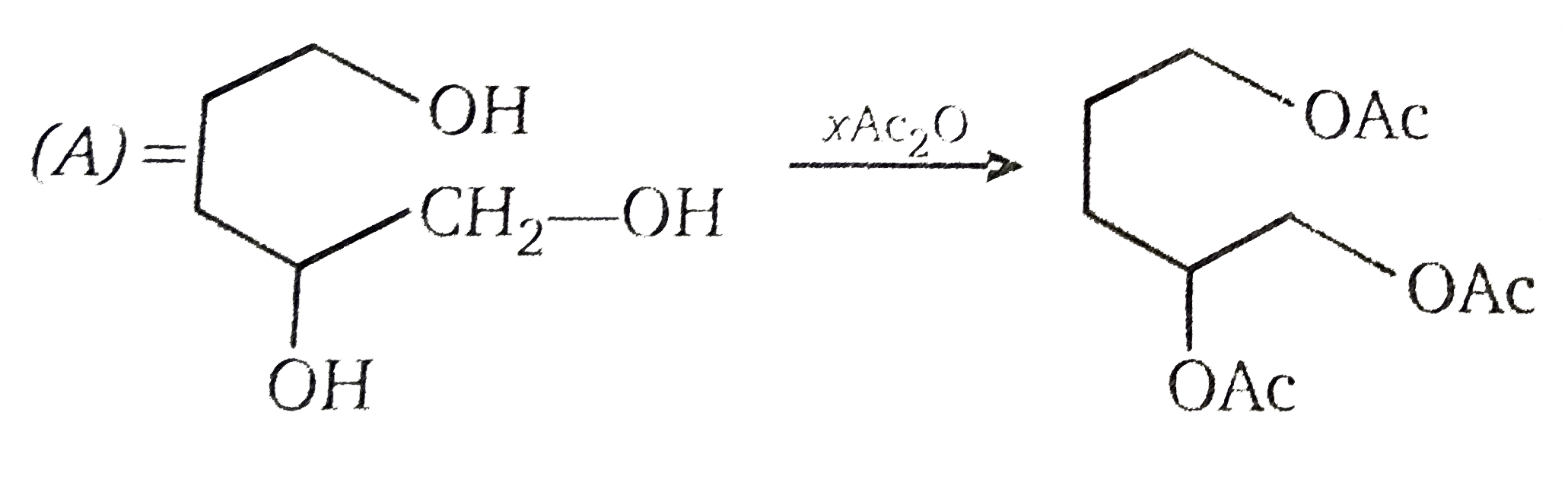
Number of alcohol is number of moles of anhydride consumed.
∴3
|
Similar Questions
Knowledge Check
An anhydride contains
Atwo unsymmetrical RCO groupsBtwo symmetrical RCO groupsCone symmetrical and one unsymmetrical groupD−COOR' groupsDuring the oxidation of Mn2+ to MnO−14 by PbO2 in acidic medium, the number of moles of acid consumed per mole of Mn2+ ion is
A4B1/2C2Dnone of these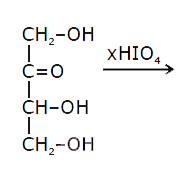
X is moles of HIO4 consumedAx = 3Bx = 2Cx = 4Dx = 1
In reaction of C6H5COCH3 with KOH+I2 to form iodoform, how many moles of "KOH" are consumed per mole of ketone "
View Solution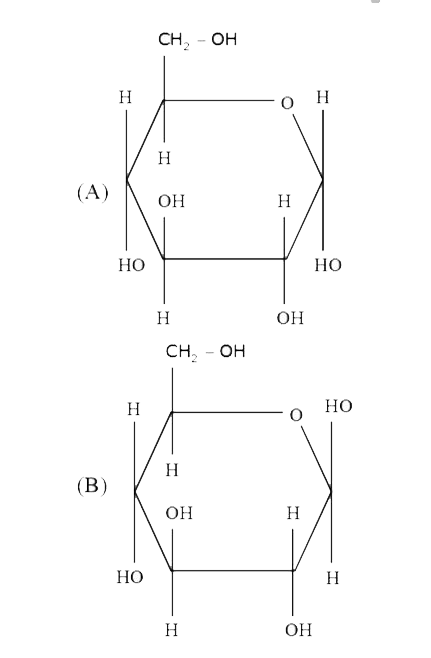
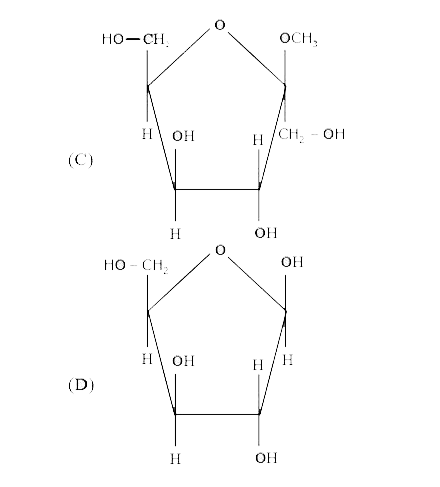
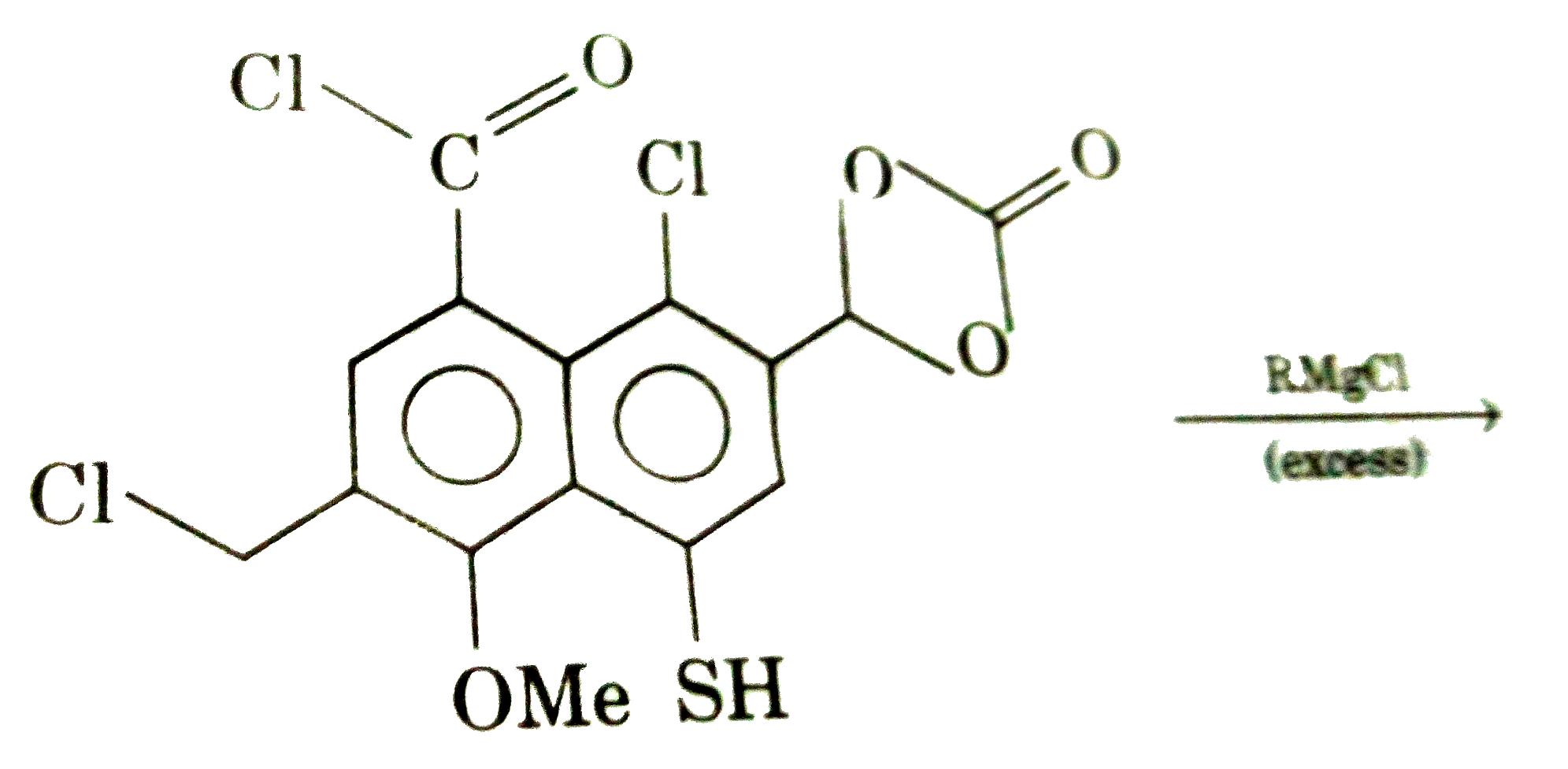
How many mole of acetic anhydride will consumed when it reacts with compound (A)
View Solution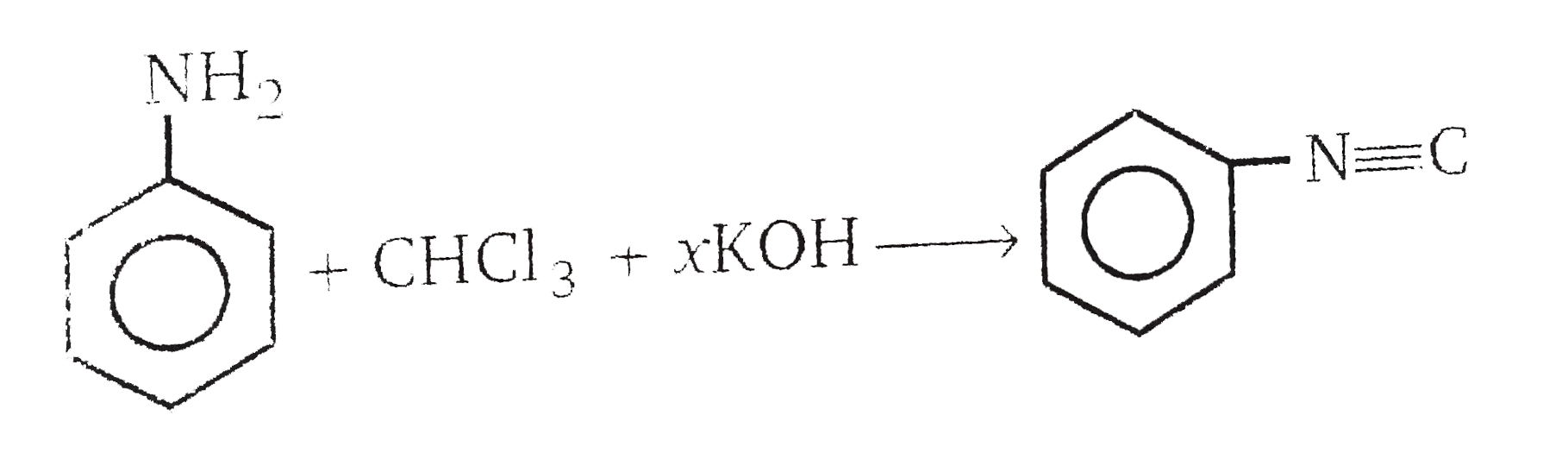
x = moles of KOH consumed is :
View SolutionR−NH2+xKOH+CHCl3→R−N≡CH where x is moles of KOH consumed, and is equal to
View SolutionR−NH2+xKOH+CHCl3→R−N≡CH where x is moles of KOH consumed, and is equal to
View Solution