CH3−CH=C=CH−CH3
To solve the nomenclature of the compound CH₃−CH=C=CH−CH₃, we will follow the IUPAC naming conventions step by step.
1. Identify the Longest Carbon Chain:
- The first step is to identify the longest continuous carbon chain in the molecule. In this case, the longest chain consists of 5 carbon atoms.
- Hint: Look for the longest sequence of carbon atoms connected by single or multiple bonds.
2. Number the Carbon Chain:
- Next, we need to number the carbon atoms in the chain. We can start numbering from either end, but we should choose the direction that gives the lowest numbers to the double bonds.
- If we number from the left, the carbon atoms are numbered as follows:
- C1: CH₃
- C2: CH
- C3: C
- C4: CH
- C5: CH₃
- Hint: Always number the chain to give the lowest possible numbers to the functional groups (in this case, the double bonds).
3. Identify the Functional Groups:
- In this molecule, we have double bonds between carbon atoms. Specifically, there are double bonds between:
- C2 and C3
- C3 and C4
- This means there are two double bonds in the compound.
- Hint: Identify the positions of the double bonds and note how many there are.
4. Assign the Correct Name:
- Since we have a 5-carbon chain (pentane) with double bonds at positions 2 and 3, we can denote this as:
- "pent" for the 5 carbon atoms,
- "2,3" for the positions of the double bonds,
- "diene" to indicate that there are two double bonds.
- Therefore, the full IUPAC name of the compound is pent-2,3-diene.
- Hint: Combine the chain name with the positions of the double bonds and the appropriate suffix for the number of double bonds.
Final Answer:
The IUPAC name of the compound CH₃−CH=C=CH−CH₃ is pent-2,3-diene.
- The first step is to identify the longest continuous carbon chain in the molecule. In this case, the longest chain consists of 5 carbon atoms.
- Hint: Look for the longest sequence of carbon atoms connected by single or multiple bonds.
- Next, we need to number the carbon atoms in the chain. We can start numbering from either end, but we should choose the direction that gives the lowest numbers to the double bonds.
- If we number from the left, the carbon atoms are numbered as follows:
- C1: CH₃
- C2: CH
- C3: C
- C4: CH
- C5: CH₃
- Hint: Always number the chain to give the lowest possible numbers to the functional groups (in this case, the double bonds).
- In this molecule, we have double bonds between carbon atoms. Specifically, there are double bonds between:
- C2 and C3
- C3 and C4
- This means there are two double bonds in the compound.
- Hint: Identify the positions of the double bonds and note how many there are.
- Since we have a 5-carbon chain (pentane) with double bonds at positions 2 and 3, we can denote this as:
- "pent" for the 5 carbon atoms,
- "2,3" for the positions of the double bonds,
- "diene" to indicate that there are two double bonds.
- Therefore, the full IUPAC name of the compound is pent-2,3-diene.
- Hint: Combine the chain name with the positions of the double bonds and the appropriate suffix for the number of double bonds.
The IUPAC name of the compound CH₃−CH=C=CH−CH₃ is pent-2,3-diene.
Topper's Solved these Questions
- NOMENCLATUREBOOK - HIMANSHU PANDEYCHAPTER - NOMENCLATUREEXERCISE - Section Ii (Q.1 To Q.25)
25 Videos
- NOMENCLATUREBOOK - HIMANSHU PANDEYCHAPTER - NOMENCLATUREEXERCISE - Section Ii (Q.26 To Q.50)
25 Videos
- NOMENCLATUREBOOK - HIMANSHU PANDEYCHAPTER - NOMENCLATUREEXERCISE - Section I (Q.76 To Q.100)
25 Videos
- ISOMERISMBOOK - HIMANSHU PANDEYCHAPTER - ISOMERISMEXERCISE - Subjective Type Problems
11 Videos
- POLYMERSBOOK - HIMANSHU PANDEYCHAPTER - POLYMERSEXERCISE - Integer Answer Type Problems
8 Videos
Similar Questions
1,2-dibromo-3-chloro-1-propene
View Solutionpentane-2,4-bione
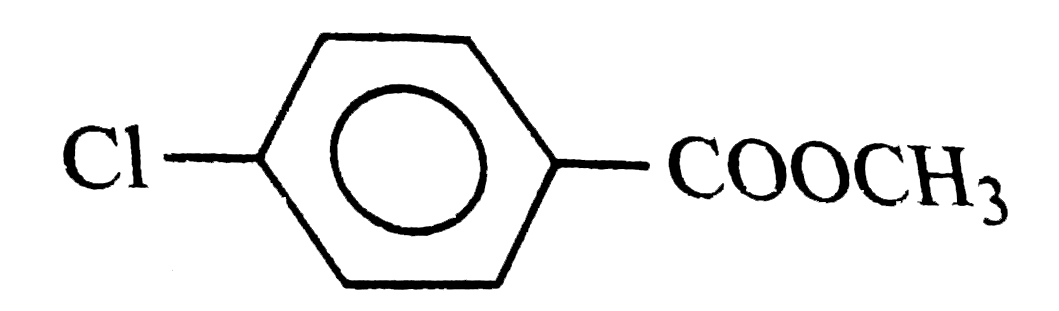
View SolutionWhat is iupac name of the following compound
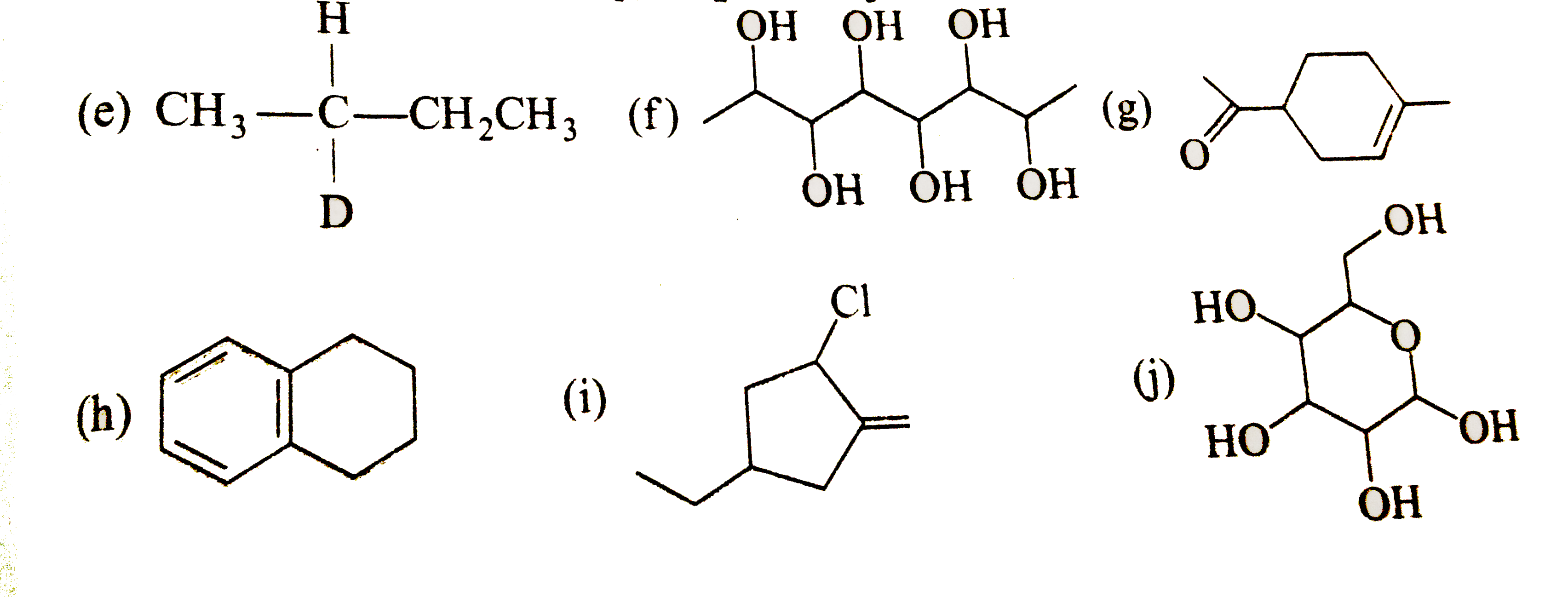
View SolutionLocate the sterogenic center (s) in each compound. A molecule may have zero, one or more sterogenic centers.
(a) CH3CH2CH2CH2CH2CH3 (b)CH3CH2OCH(CH3)CH2CH3
( c) (CH3)2CHCH(OH)CH(CH3)2
(d)(CH3)2CHCH2CH(CH3)CH2CH(CH3)CH(CH3)CH2CH3
View SolutionThe number of condensation copolymers among the following is_________.
SBR, polyester, bakelite, nylon-6, PVC, starch, nylon-6,6, glyptal, natural rubber.
View SolutionName of the following as substituted derivatives of ethylene.
(a) CH3−CH=CH−CH3
(b) CI2C=CHCI
(c) (CH3)2C=CH2
(d) (CH2=CH)2C=CH2
(e) (CH2=CH)−CH=CH−(CH=CH2)
View SolutionWrite the IUPAc name of the following compound :
(a) CH3−CH2−CH3∣CH−CH=CH−CH2−CH3
(b) CH3−CH2−CH3∣CH−CH2−CH3∣CH−CH∣CH2∣CH2∣CH−CH3∣CH3−CH=CH3∣C−CH3
View SolutionFor a molecule given select the correct statement (s). CH_(3)-CH=CH-CH=C=C=CH-CH=C=CH-CH_(3)
View SolutionIUPAC name is CH3−CH=CH−CH=CH2
View SolutionCH_(3)-underset(CH_(3))underset(|)(CH)-CH=CH-CH_(2)-overset(O)overset(||)(C)-OH
View SolutionO ∣∣CH3C=CH−C=CH−C−CH3 | | CH3 Cl
View SolutionCH_(2)=CH-C-=C-CH=CH-CH_(3)
View Solutionनिम्नलिखित यौगिक का सही नाम है -
CH3 |CH3CH2−C=CH−CH−CH2−CH3 |CH3CH2−CH−CH2−CH2−CH3
View Solutionनिम्नलिखित यौगिकों के IUPAC नाम लिखिए-
(i) CH3 |CH3−CH2−CH−CH−C−CH3 | | | CH3 I CH3
(ii) Cl |CH3−CH2−CH−CH−C−CH3 | | | CH3 OH CH3
View SolutionCH3CH2CH=CH2 and CH3CH=CH−CH3 show
View Solution

