The following has lowest pH
A
MgSO4
B
MgCO3
C
NaCl
D
Sodium oxalate
Video Solution
Text Solution
The correct Answer is:A
Due to cationic hydrolysis
|
Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - NARAYNACHAPTER - IONIC EQUILIBRIUMEXERCISE - Level-I
49 Videos
- IONIC EQUILIBRIUMBOOK - NARAYNACHAPTER - IONIC EQUILIBRIUMEXERCISE - Level-II
48 Videos
- IONIC EQUILIBRIUMBOOK - NARAYNACHAPTER - IONIC EQUILIBRIUMEXERCISE - Level-VI (H.W)
35 Videos
- HYDROGEN & ITS COMPOUNDSBOOK - NARAYNACHAPTER - HYDROGEN & ITS COMPOUNDSEXERCISE - LEVEL-4
21 Videos
- PERIODIC TABLE BOOK - NARAYNACHAPTER - PERIODIC TABLE EXERCISE - All Questions
568 Videos
Similar Questions
Knowledge Check
Which of the following has lowest pKa value ?
A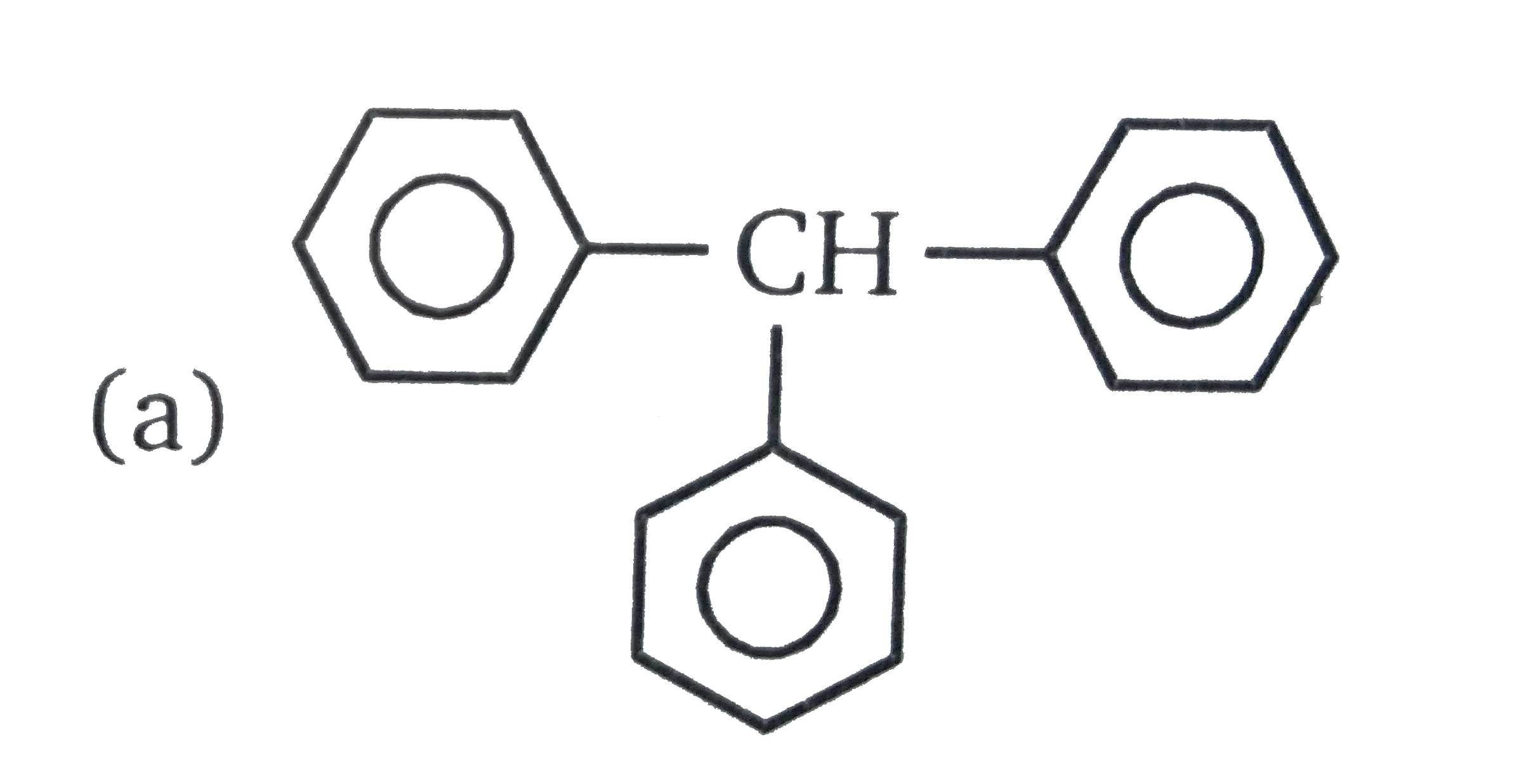 B
B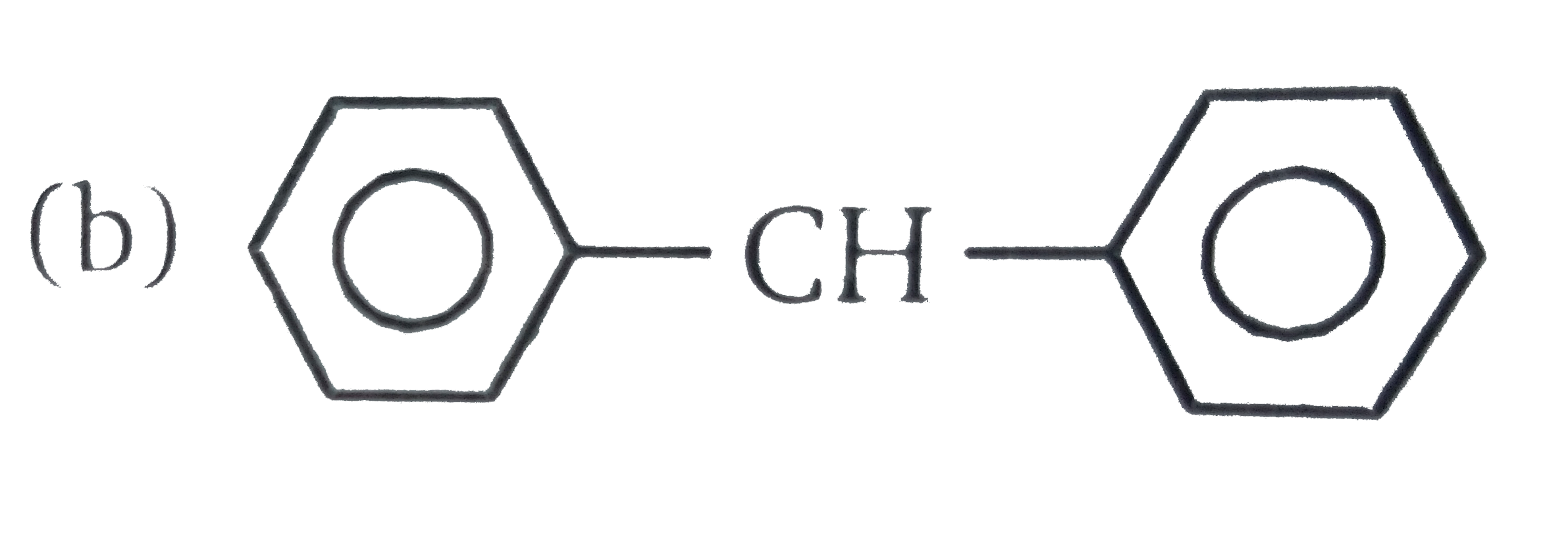 C
C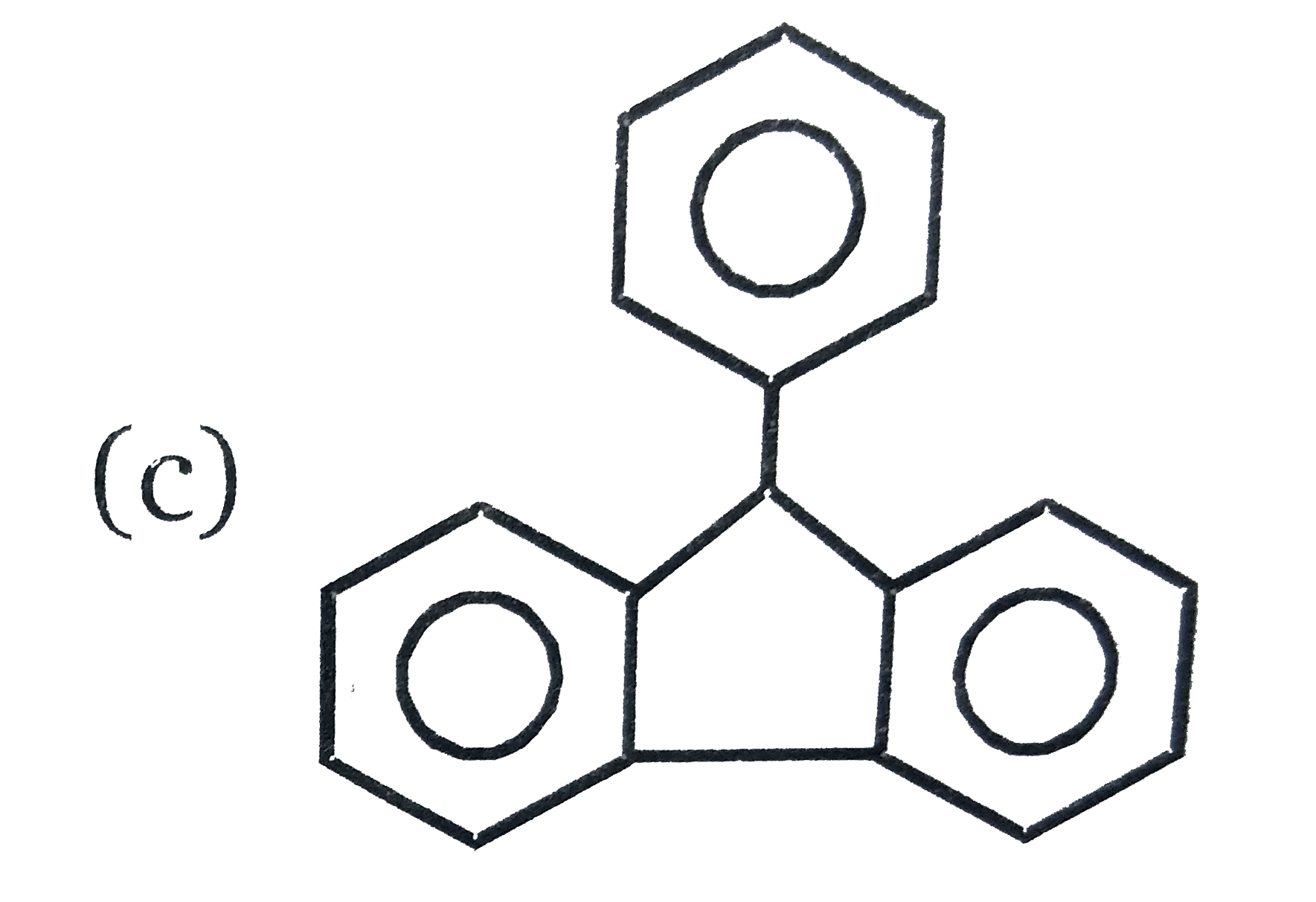 D
D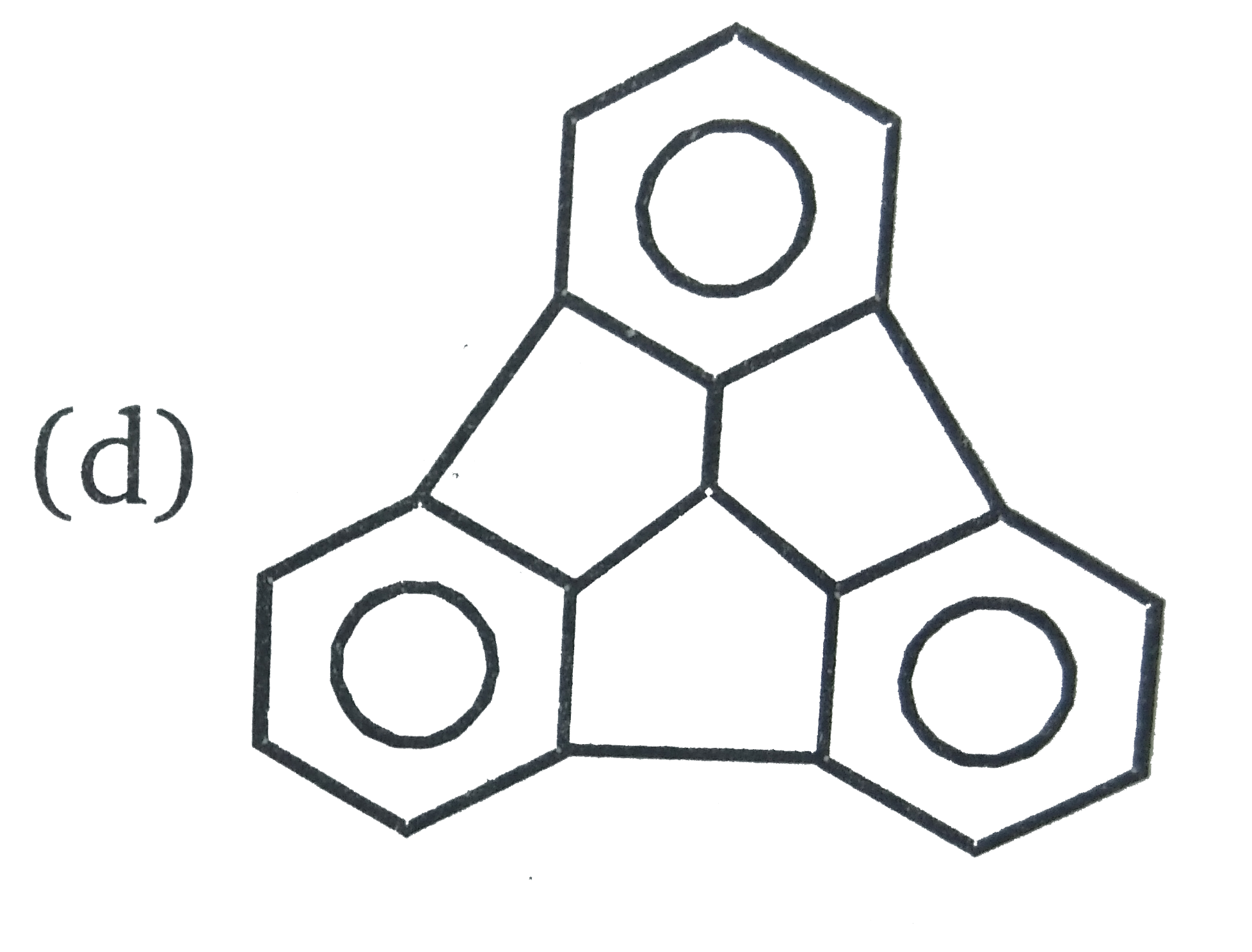
Which of the following has lowest B.P
ACH3−CH2−CH2−NH2BCH3−CH∣NH2−CH3CC2H5−NH−CH3D(CH3)3NWhich one of the following has lowest m.pt ?
ABBTICAlDGa
Among the following which has lowest pKa value?
View SolutionAmong the following which has lowest pKa values ?
View SolutionWhich of the following has lowest dipole moment?
View SolutionWhich of the following has lowest percentage of carbon?
View SolutionWhich of the following has the lowest pKa value?
View Solution

