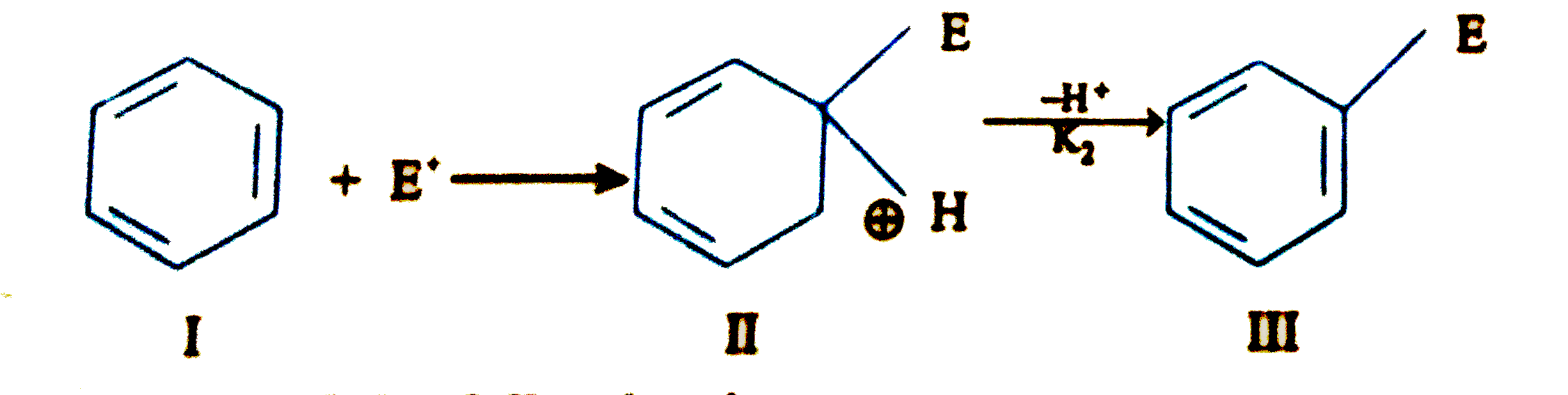
The mechanism of an electrophilic substitution reaction is
Which of the following is not ture.
A
Increasing the resonance of II will increases rate of reaction
B
increasing the density of electron in I will increase the rate of reaction.
C
Treatment of base in step-II will increase the rate
D
Temperature increasing will accelerate the rate of reaction.
Text Solution
The correct Answer is:C
H⊕ is lost in the fast step.
|
Similar Questions
Knowledge Check
The example of an electrophilic substitution reaction is
A B
B CH2C=CH−CH3HBr−−−−→peroxide
CH2C=CH−CH3HBr−−−−→peroxide
Br2−CH2−CH2−CH3DH2CH2−CH2−O∣∣C−CH3Correct order of electrophilic substitution reaction is :
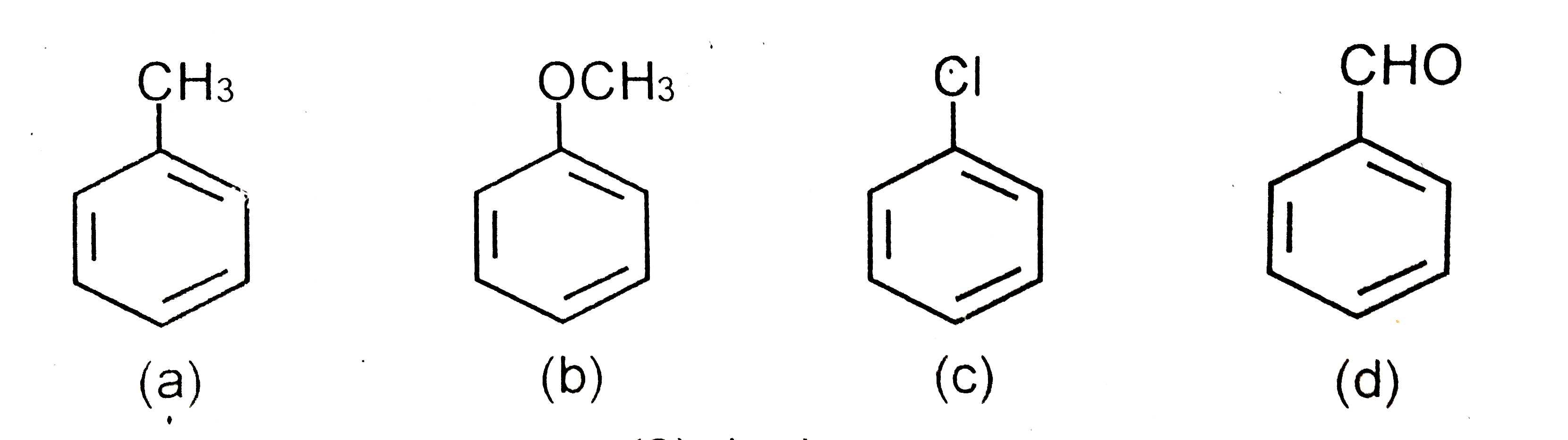 Aa>b>c>dBd>b>a>cCb>a>c>dDb>a>d>c
Aa>b>c>dBd>b>a>cCb>a>c>dDb>a>d>cFor an electrophilic aromatic substitution reaction
AChlorine is o-p directing group and also electron releasing groupBChlorine is o-p directing group and also electron withdrawing groupCChlorine is meta directing group and also electron releasing groupDChlorine is meta directing group and also electron withdrawing group
Electrophilic Substitution Reactions|Summary
View SolutionElectrophilic Substitution Reactions|Summary
View SolutionElectrophilic Substitution Reactions|Summary
View SolutionIn electrophilic substitution reaction nitrobenzene is
View SolutionWhich of the following is an electrophilic substitution reaction?
View Solution

