For a typical nucleophilic aromatic subsitution reaction to take place.
(1) Nucleophilic atom should be of oxygen, nitrogen or sulphur.
(2) Leaving group should be halide .
(3) There should be strong electron withdarwing at ortho and para position to leaving group.
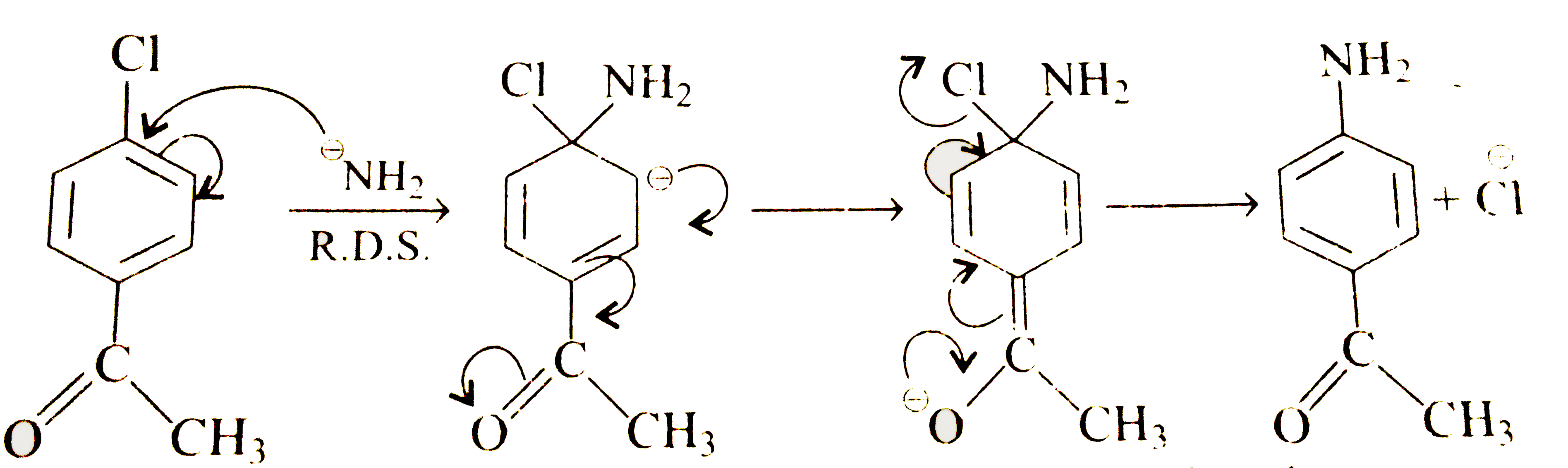
Fastest nucleophilic aromatic substitution reaction take place in :
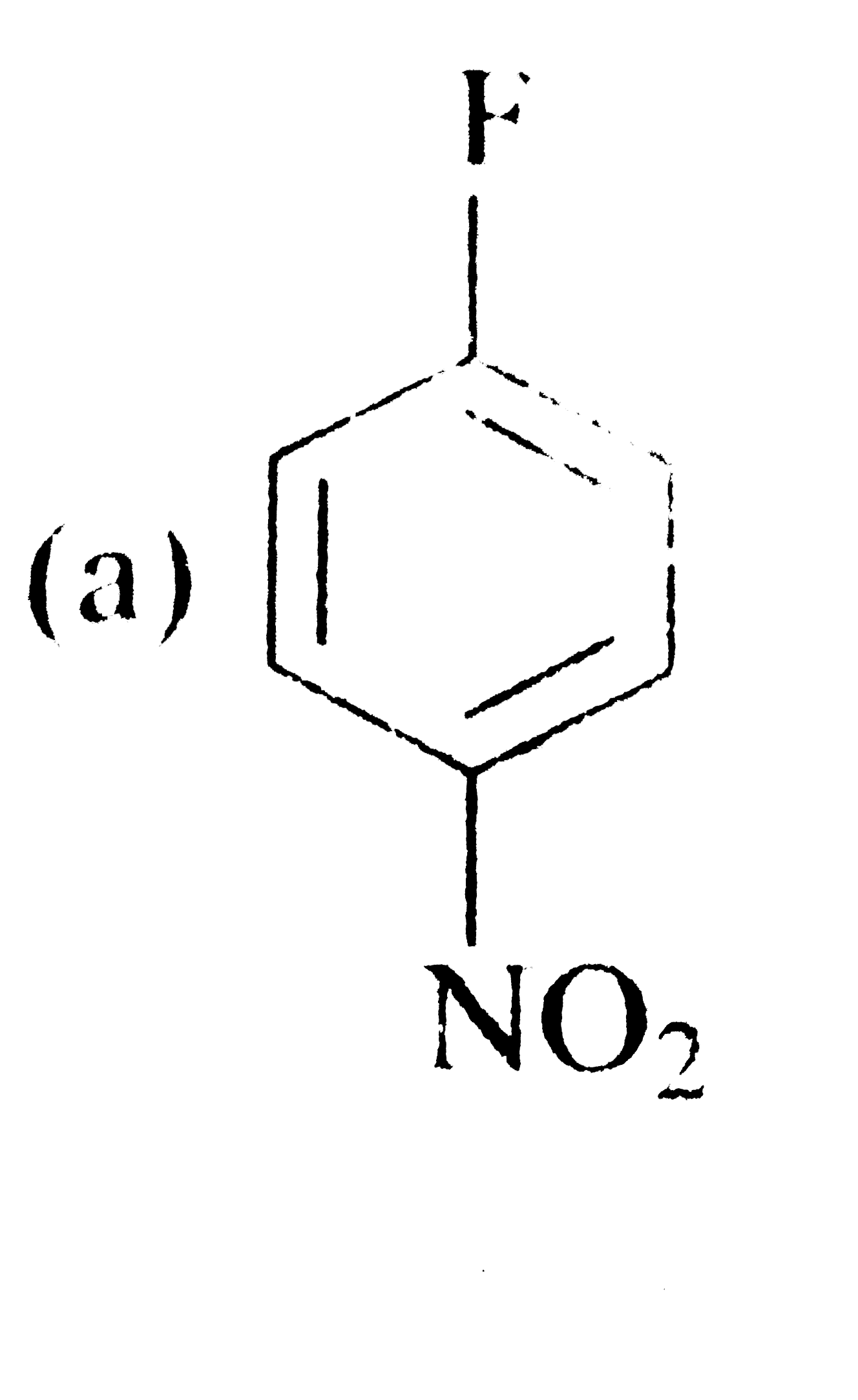
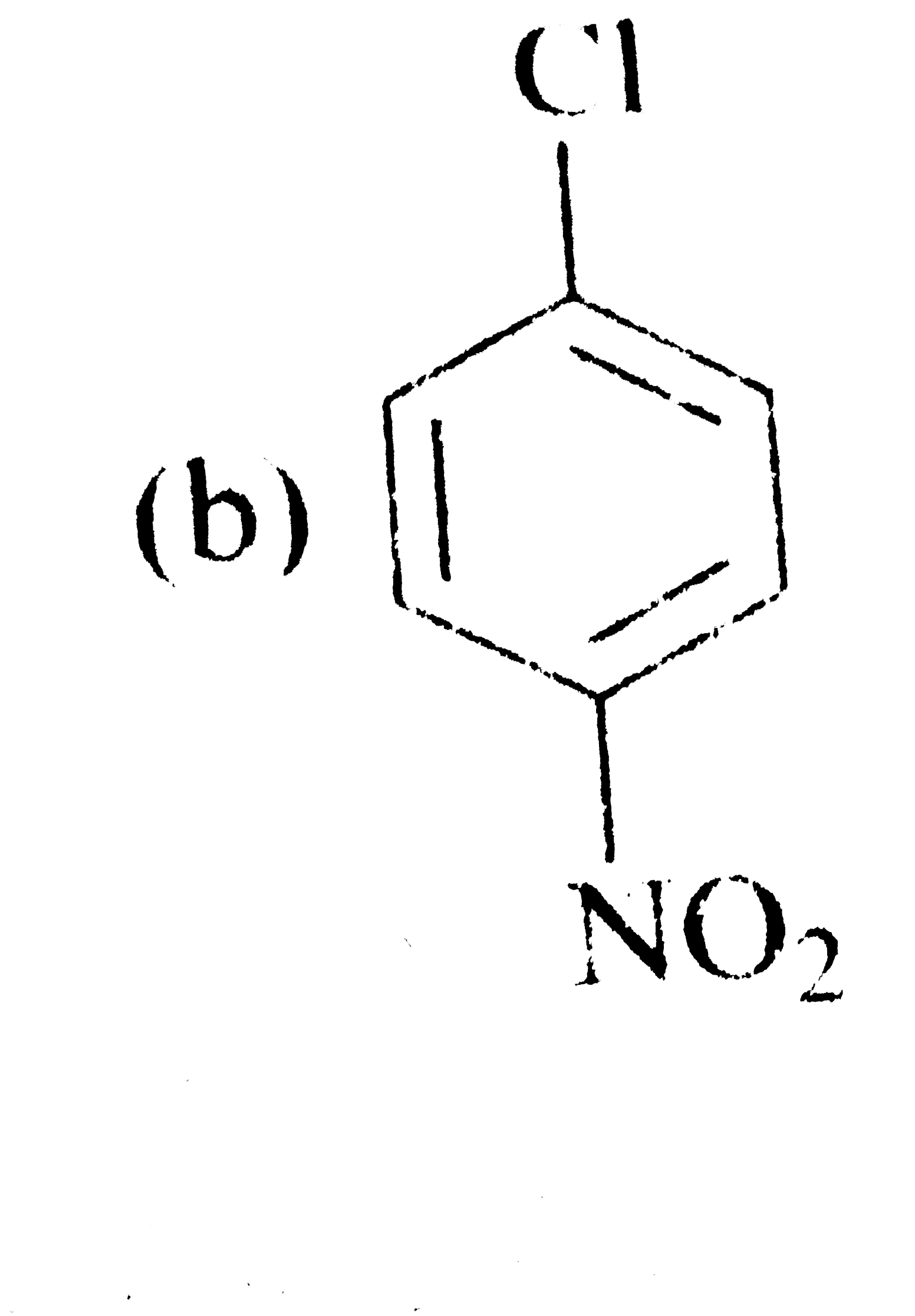
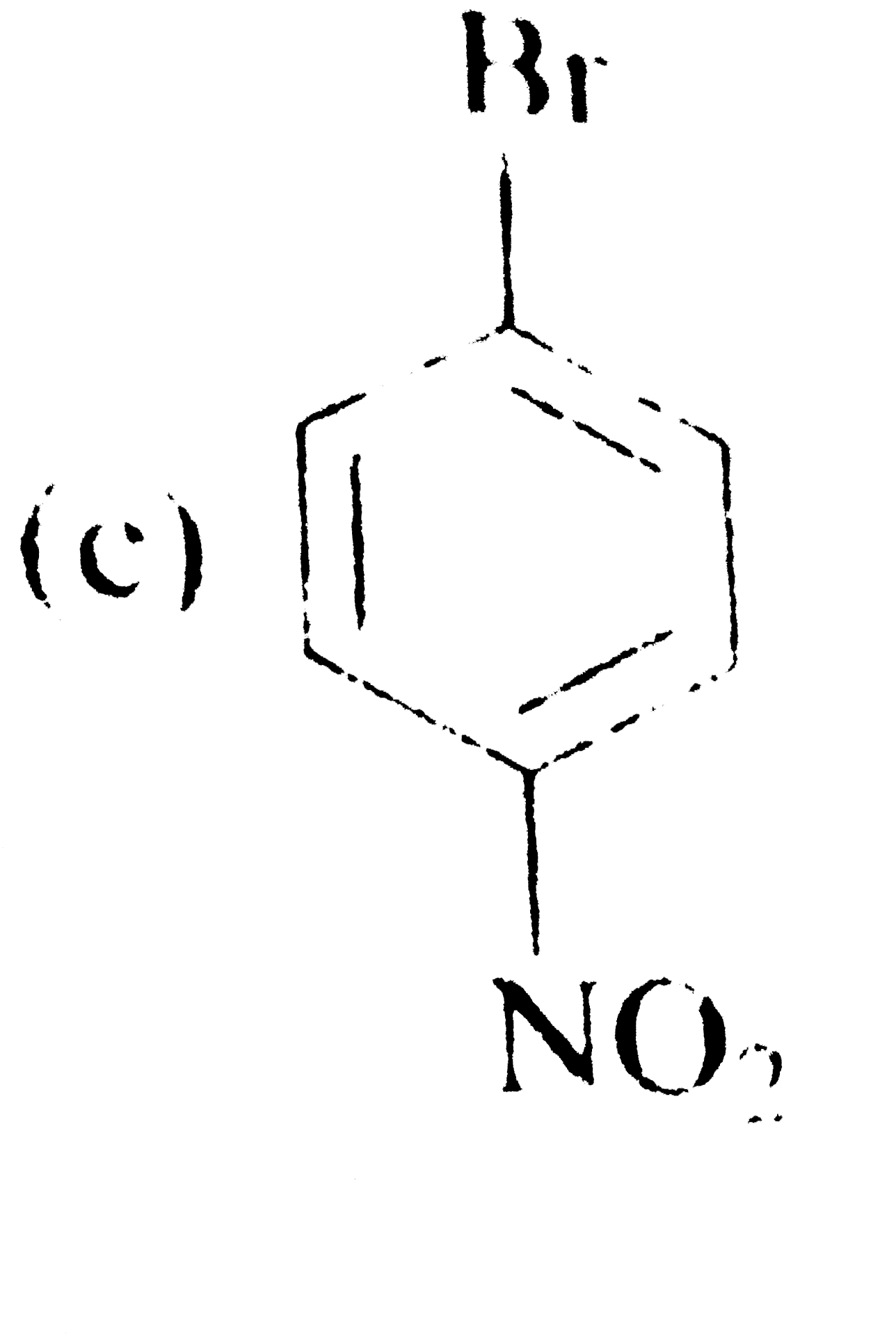
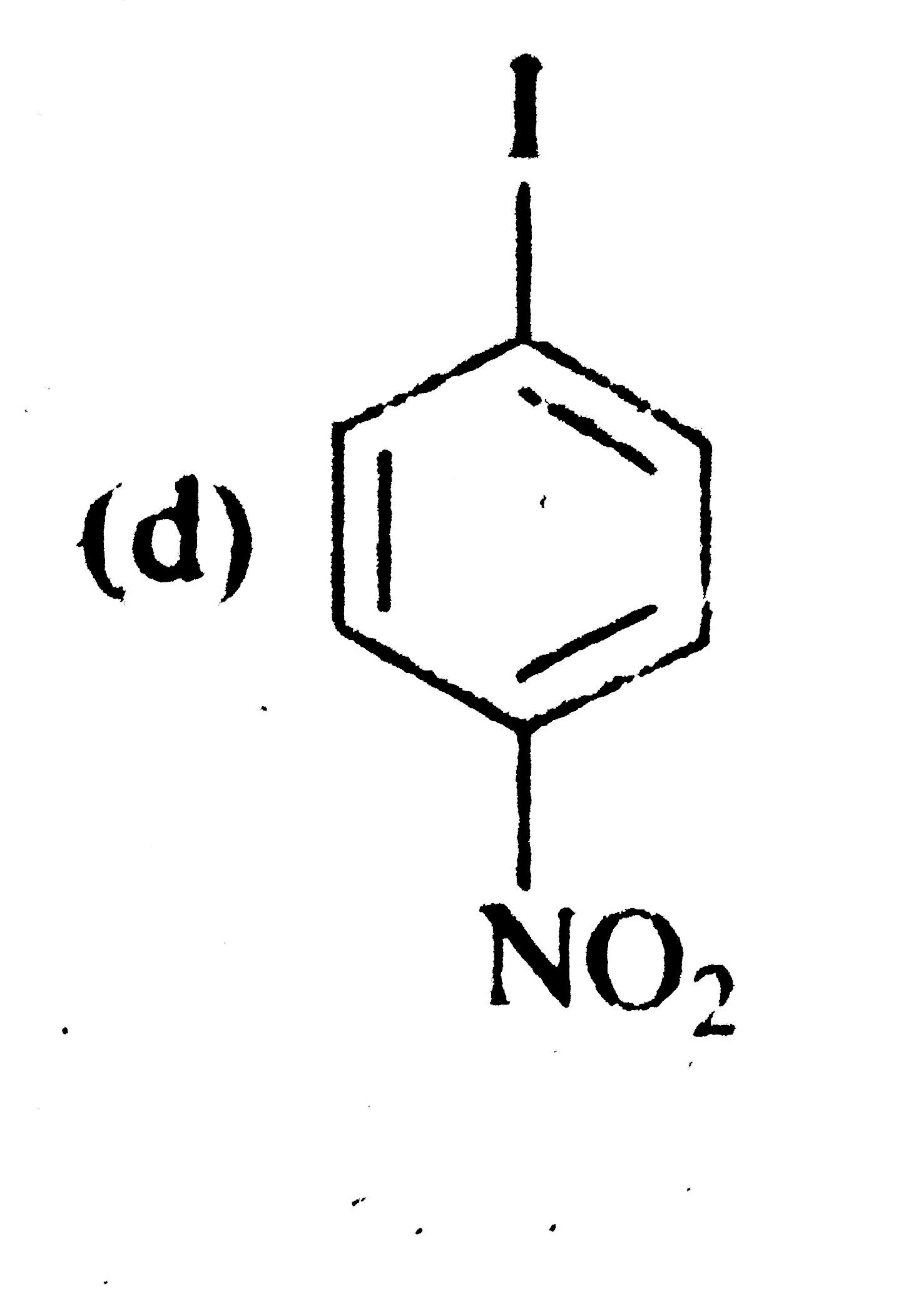
The correct Answer is:A
Step by step video, text & image solution for For a typical nucleophilic aromatic subsitution reaction to take place. (1) Nucleophilic atom should be of oxygen, nitrogen or sulphur. (2) Leaving group should be halide . (3) There should be strong electron withdarwing at ortho and para position to leaving group. Fastest nucleophilic aromatic substitution reaction take place in : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Nucleophilic Substitution Introduction and Leaving Group
View SolutionIn nucleophilic substitution reaction, the order of halogens as leaving group should be :
View SolutionWhich is the best leaving group in a nucleophilic substitution reaction?
View SolutionStatement-1: Aldehyde and ketone give nucleophilic addition reactions with carbon oxygen, nitrogen and sulphur nucleophiles. Statement-2: Sulphite ion is ambident nucleophile.
View SolutionEffect of Solvent on Nucleophilicity and basicity | Leaving Group and Leaving Group Tendency
View SolutionThe correct order of leaving group ability in a nucleophilic substitution reaction is
View SolutionWhat is meant by 'leaving group' in a nucleophillic substitution reaction.
View SolutionSolvent Effect on Nucleophilicity || Nucleophilicity Comparison Leaving Groups and Their Comparison Leaving Group Nature
View Solution

