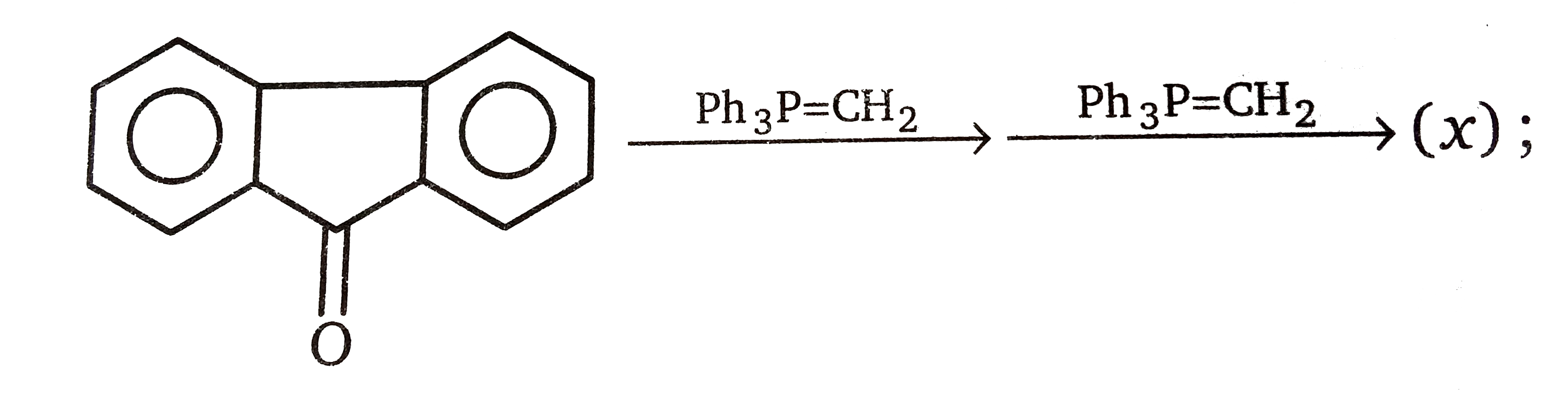
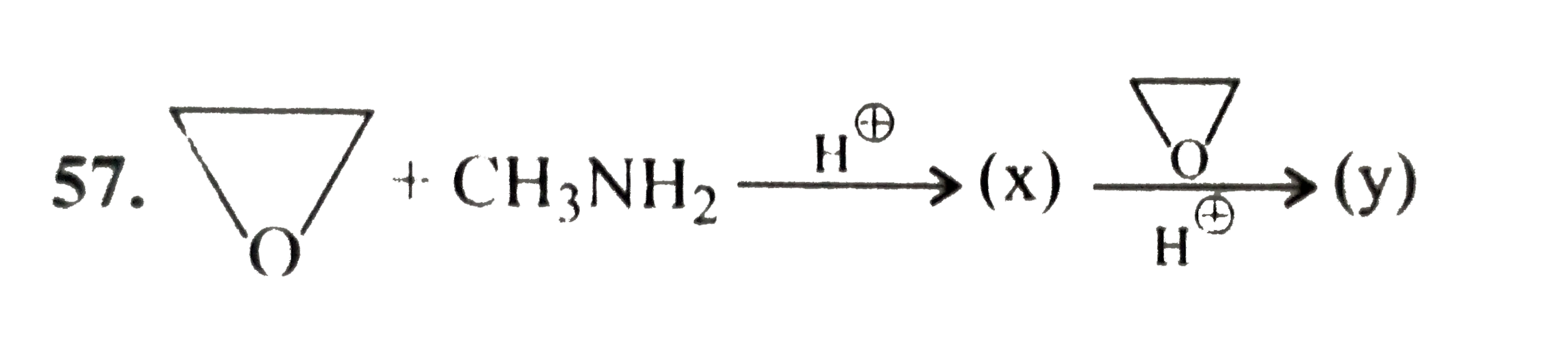 Product (x) is:
Product (x) is:
A
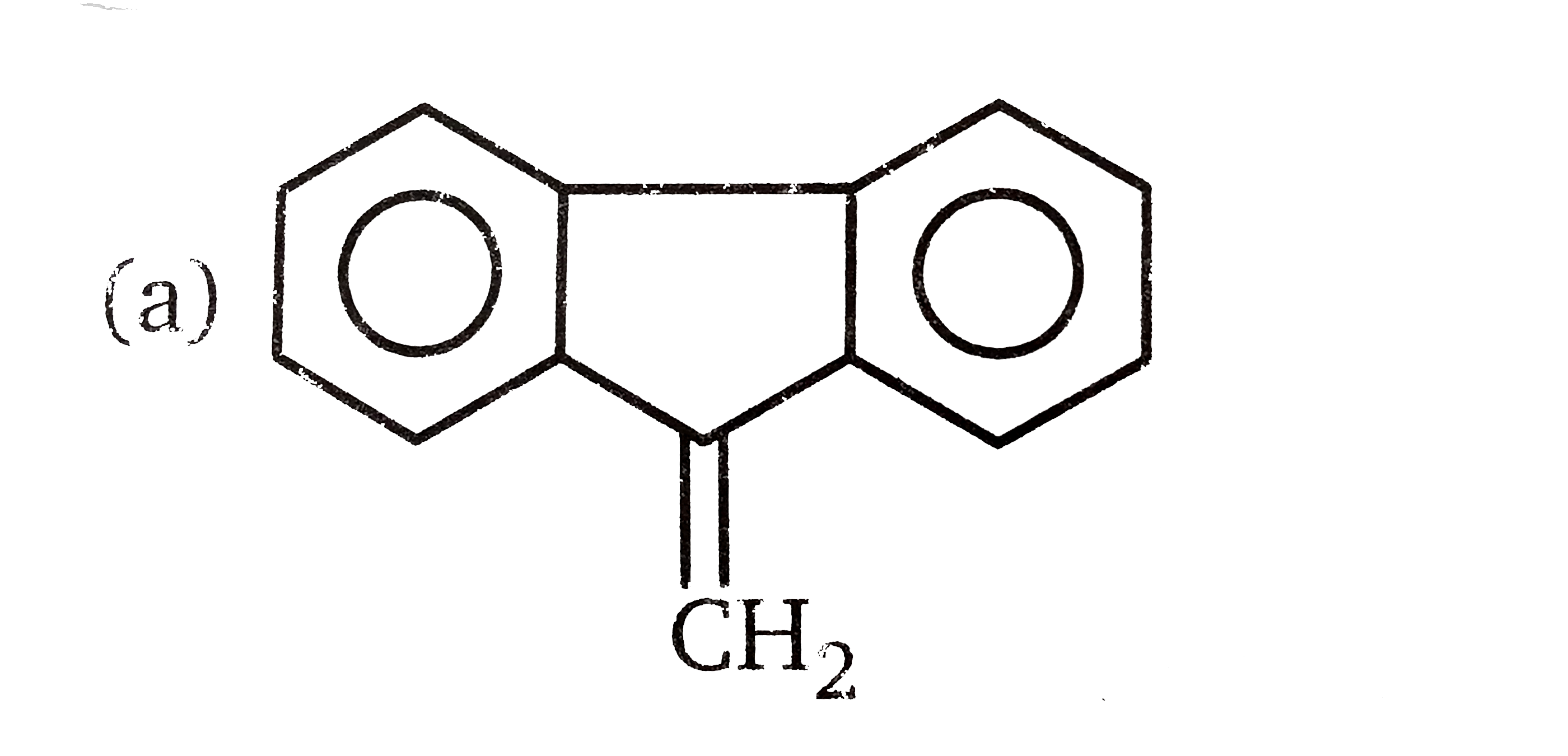
B
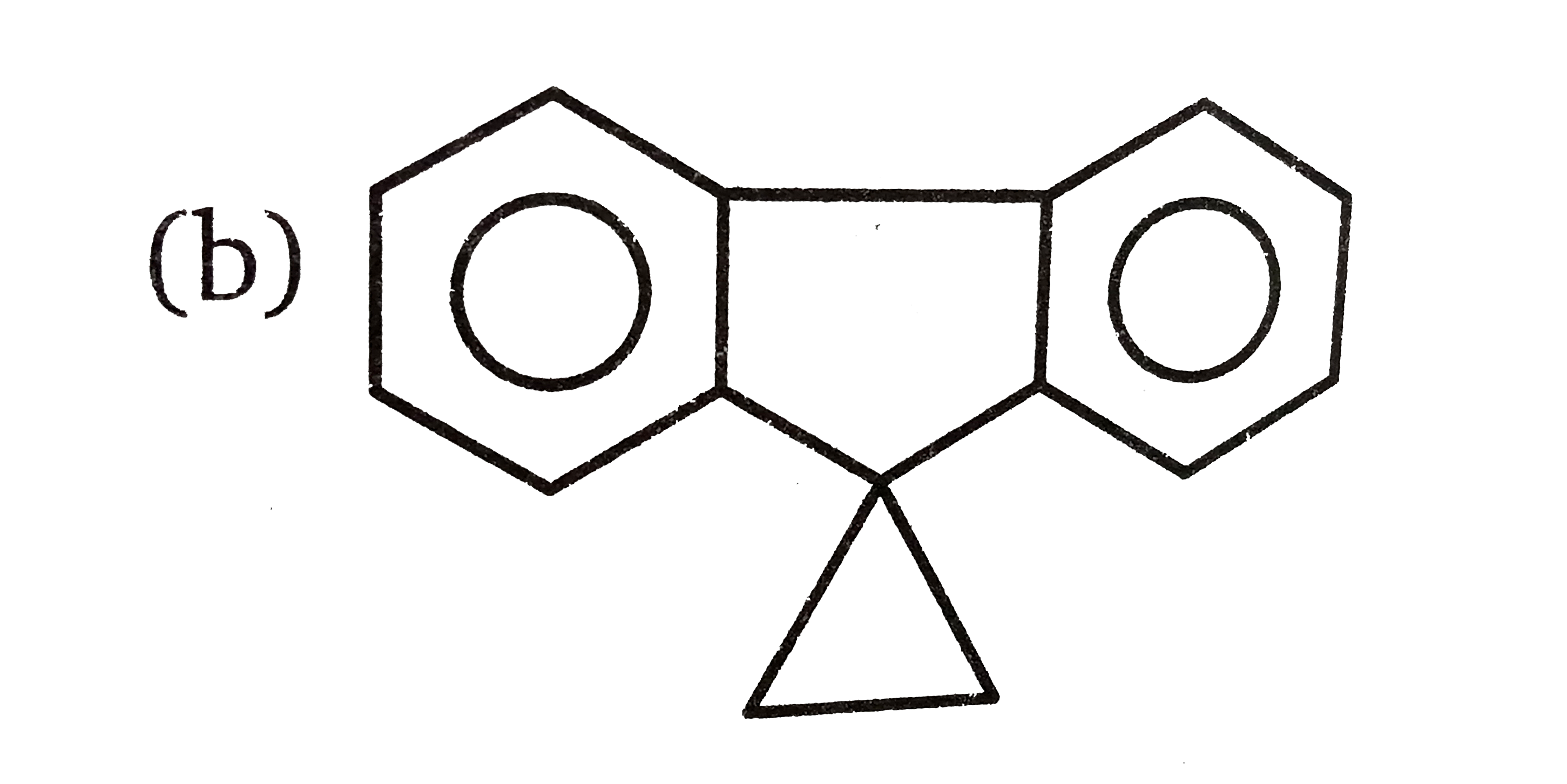
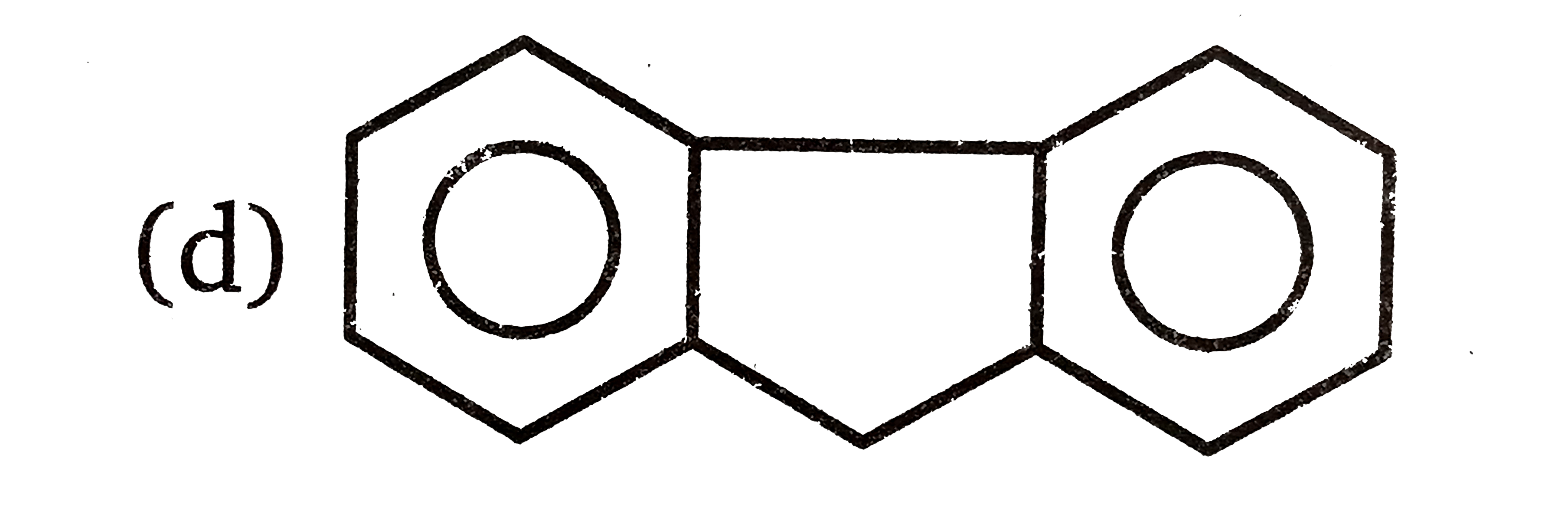
C
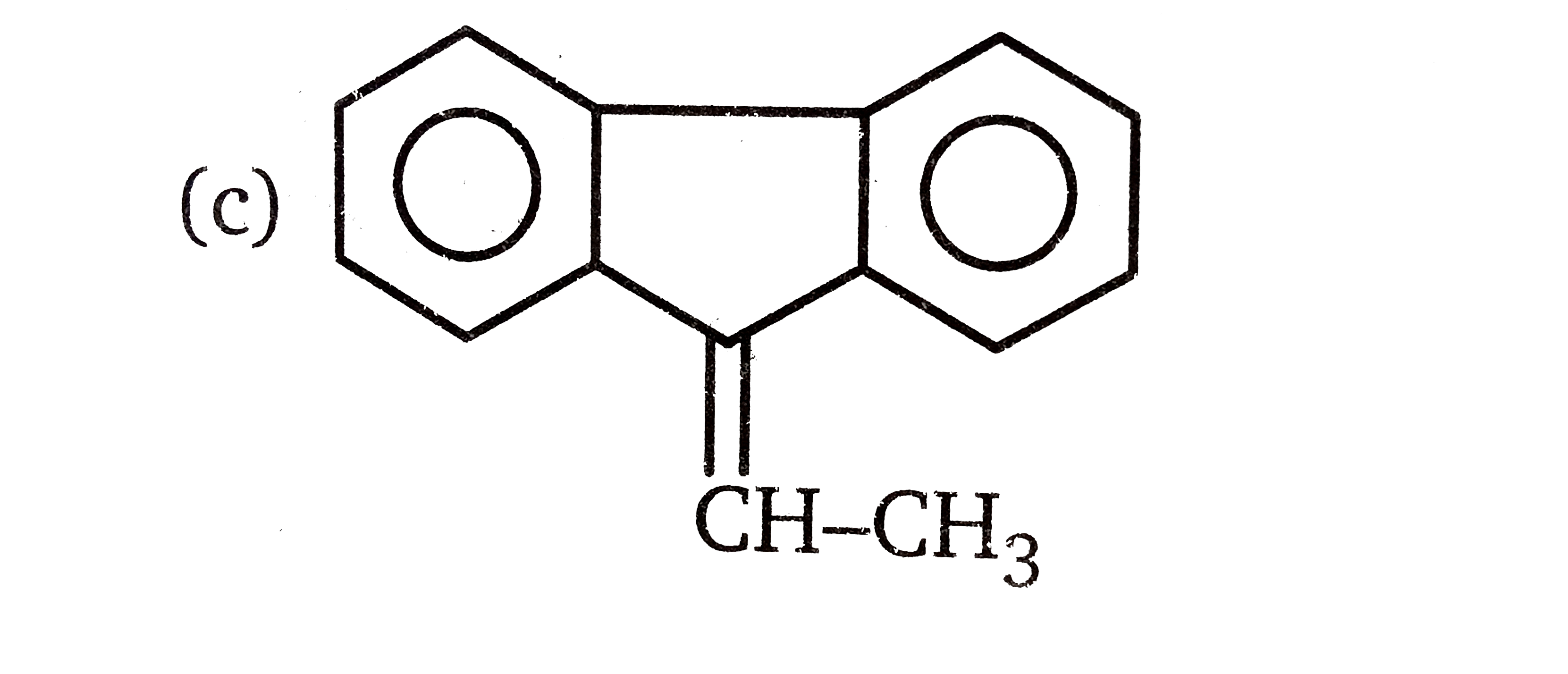
D
Video Solution
Text Solution
The correct Answer is:b
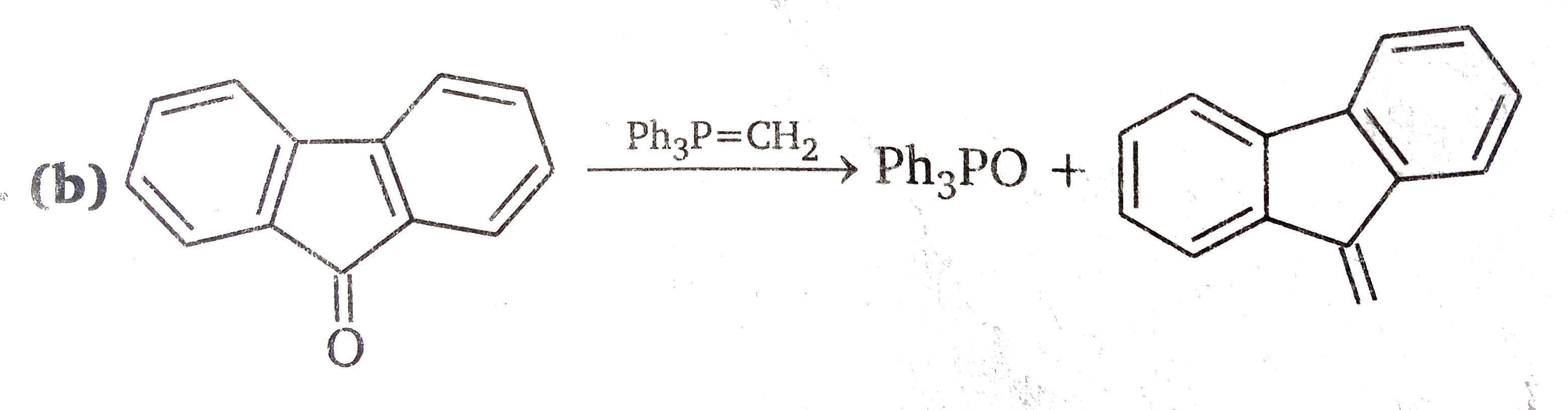
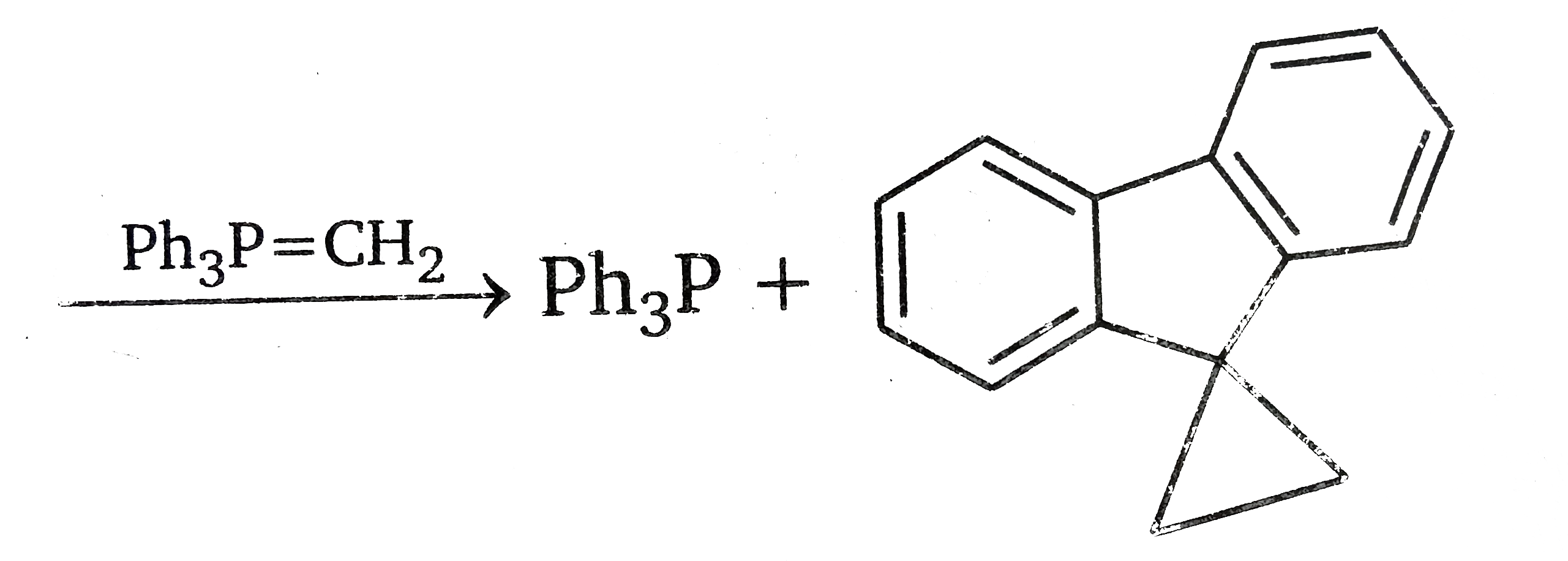
Since there is no carbonyl group in the first product, the double bond of the exocyclic methaylene gorup has reacted with methylenephosphorane. We may therefore propose a mechanism based on the assumption that the exocyclic double bond behaves like the double bond in a carbonyl group.The latter reaction is.
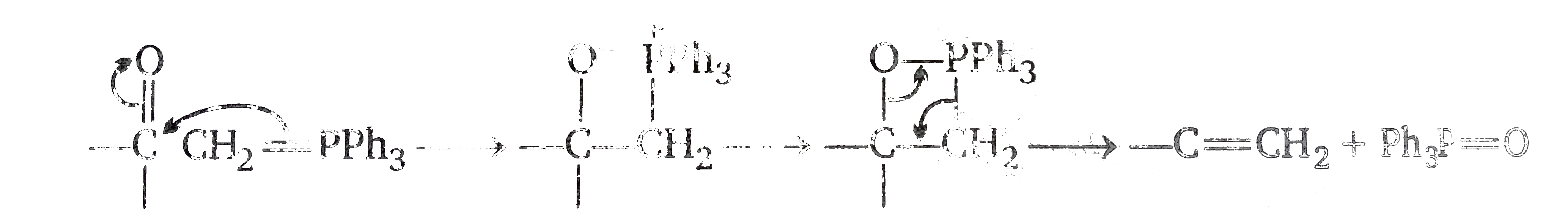 However in the case under consideration, there is no oxygen atom, phosphine and not phosphine oxide is eliminated, and a singlr and not a double bond is formed with the methtylene group. This can be accomodatd by replacing O− by >C− (of the five- membered ring). and then as follows.
However in the case under consideration, there is no oxygen atom, phosphine and not phosphine oxide is eliminated, and a singlr and not a double bond is formed with the methtylene group. This can be accomodatd by replacing O− by >C− (of the five- membered ring). and then as follows. 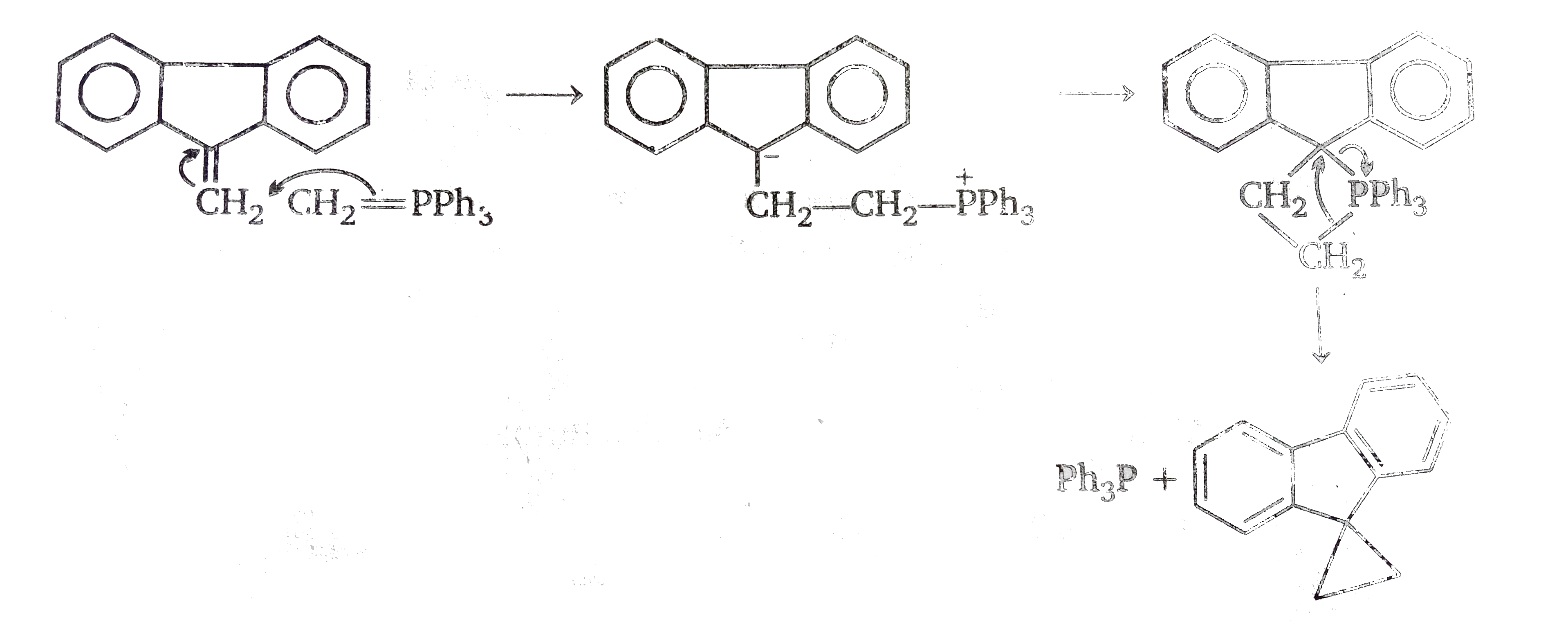
In this sequence,we have propopsed a complete parallel with the carbonyl group and this results in the formation of a spir-compound conataing phosphours in one ring. The final prodced is also a spiro-compound, but since this conatins a three memerbed ring. it could be argued that this is less stable than is precuros - which contains a four -membered the mechanism involving neighbourng group participation i.e.
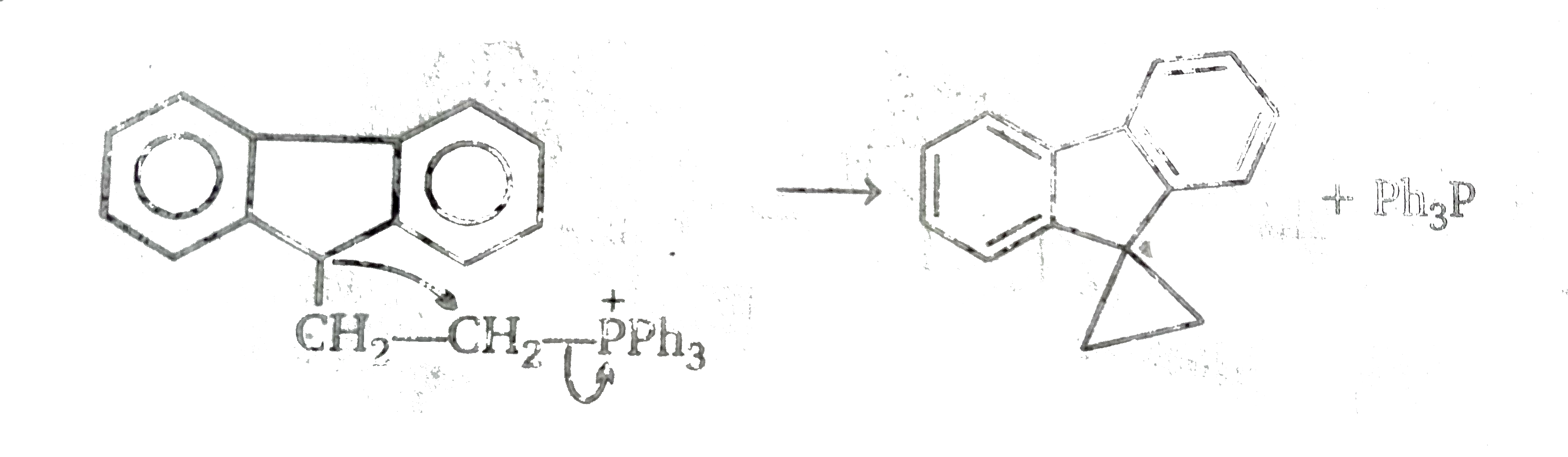
This route would be energetically more favourable than the other one and so is more likely one.
|
Topper's Solved these Questions
- HYDROCARBONS (ALKENES)BOOK - MS CHOUHANCHAPTER - HYDROCARBONS (ALKENES)EXERCISE - Level 1 (Q.1 To Q.30)
1 Video
- HYDROCARBONS (ALKENES)BOOK - MS CHOUHANCHAPTER - HYDROCARBONS (ALKENES)EXERCISE - Level 1 (Q.61 To Q.90)
2 Videos
- HYDROCARBONS (ALKANES)BOOK - MS CHOUHANCHAPTER - HYDROCARBONS (ALKANES)EXERCISE - Level 1 (Q.31 To Q.50)
1 Video
- HYDROCARBONS (ALKYNES)BOOK - MS CHOUHANCHAPTER - HYDROCARBONS (ALKYNES)EXERCISE - Level 1 (Q.1 To Q.30)
2 Videos
Similar Questions
Knowledge Check
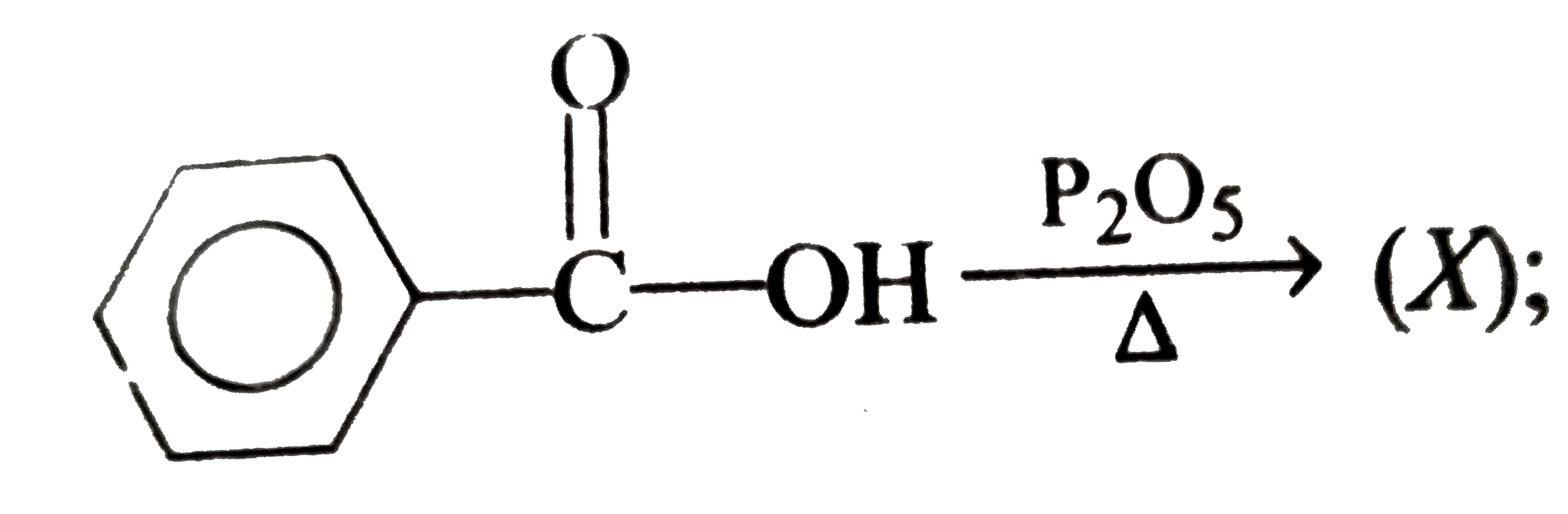 Identify Product (X) :A
Identify Product (X) :A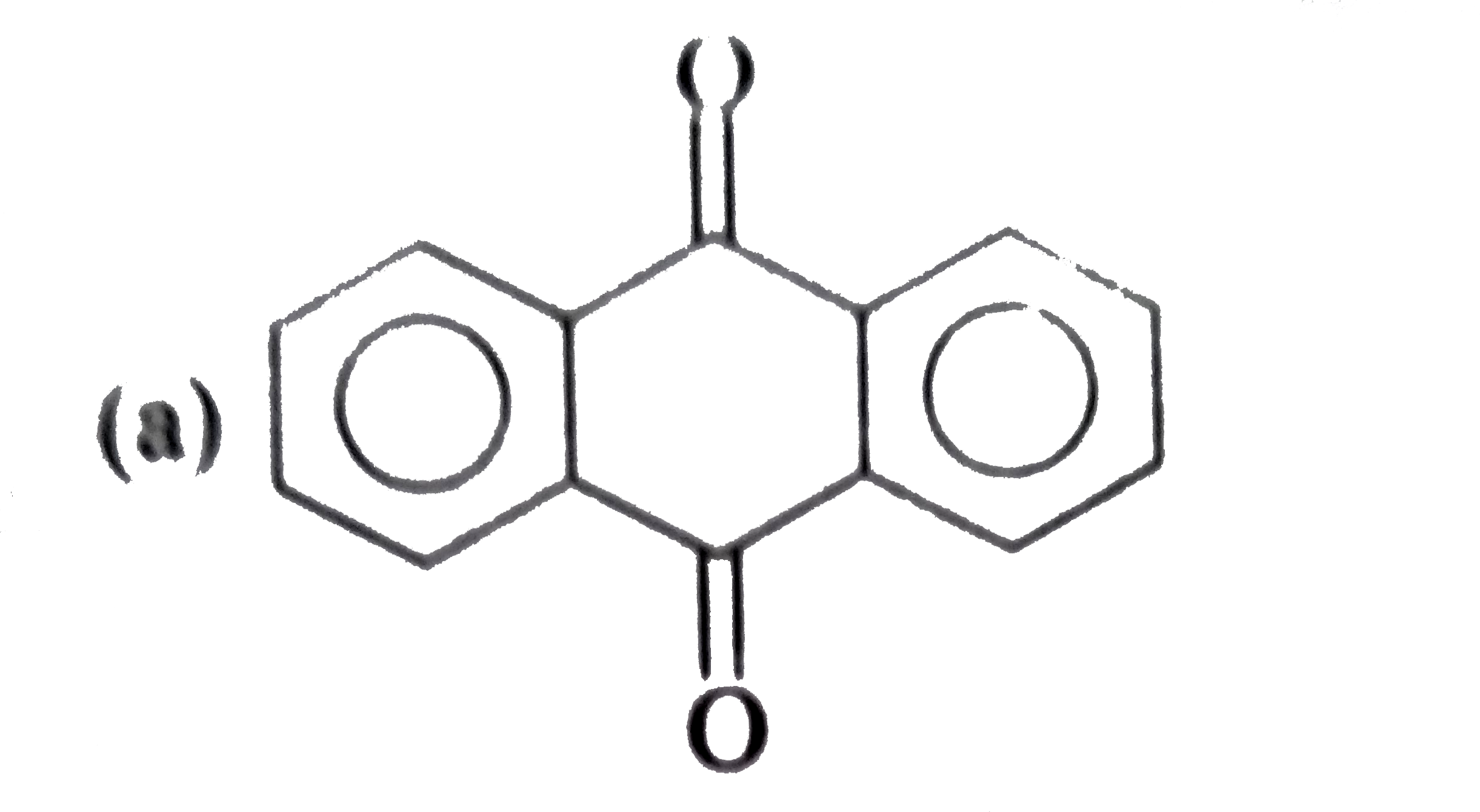 B
B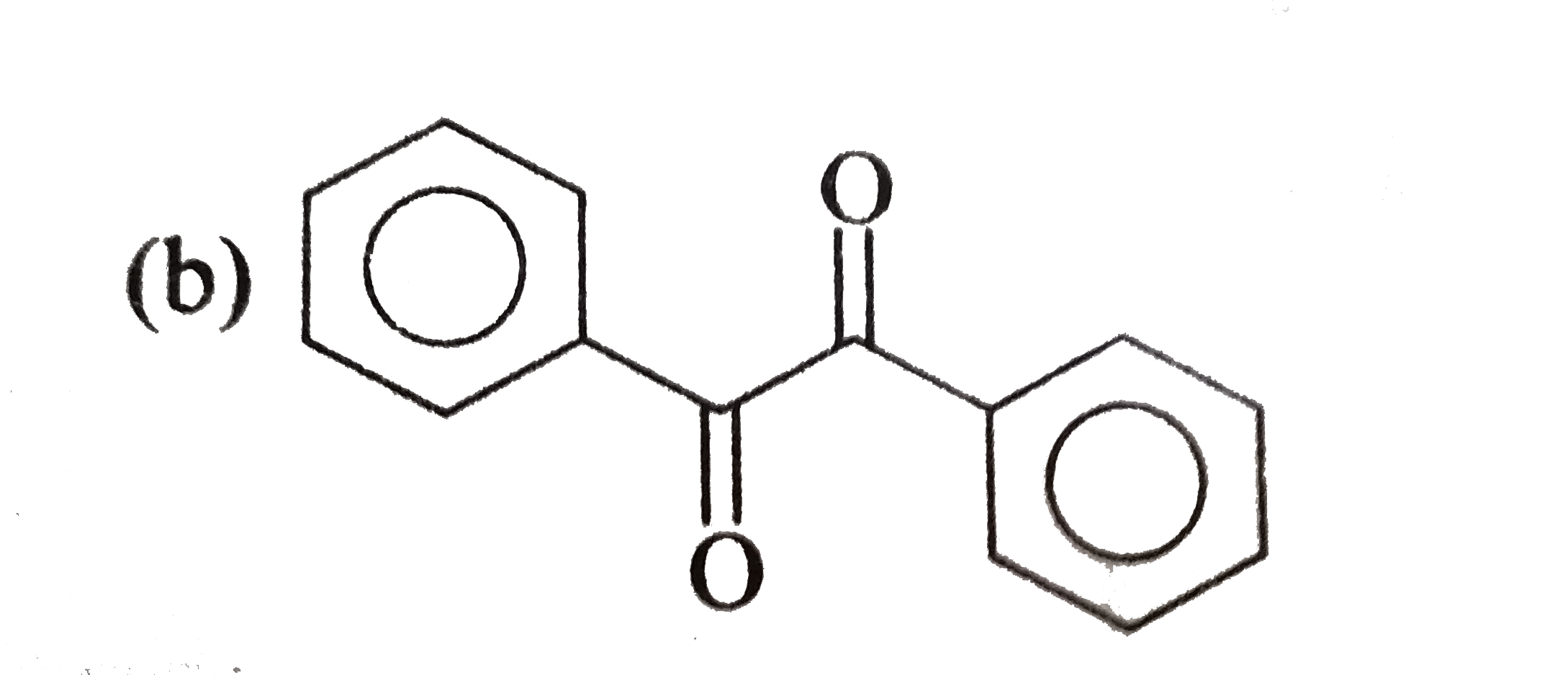 C
C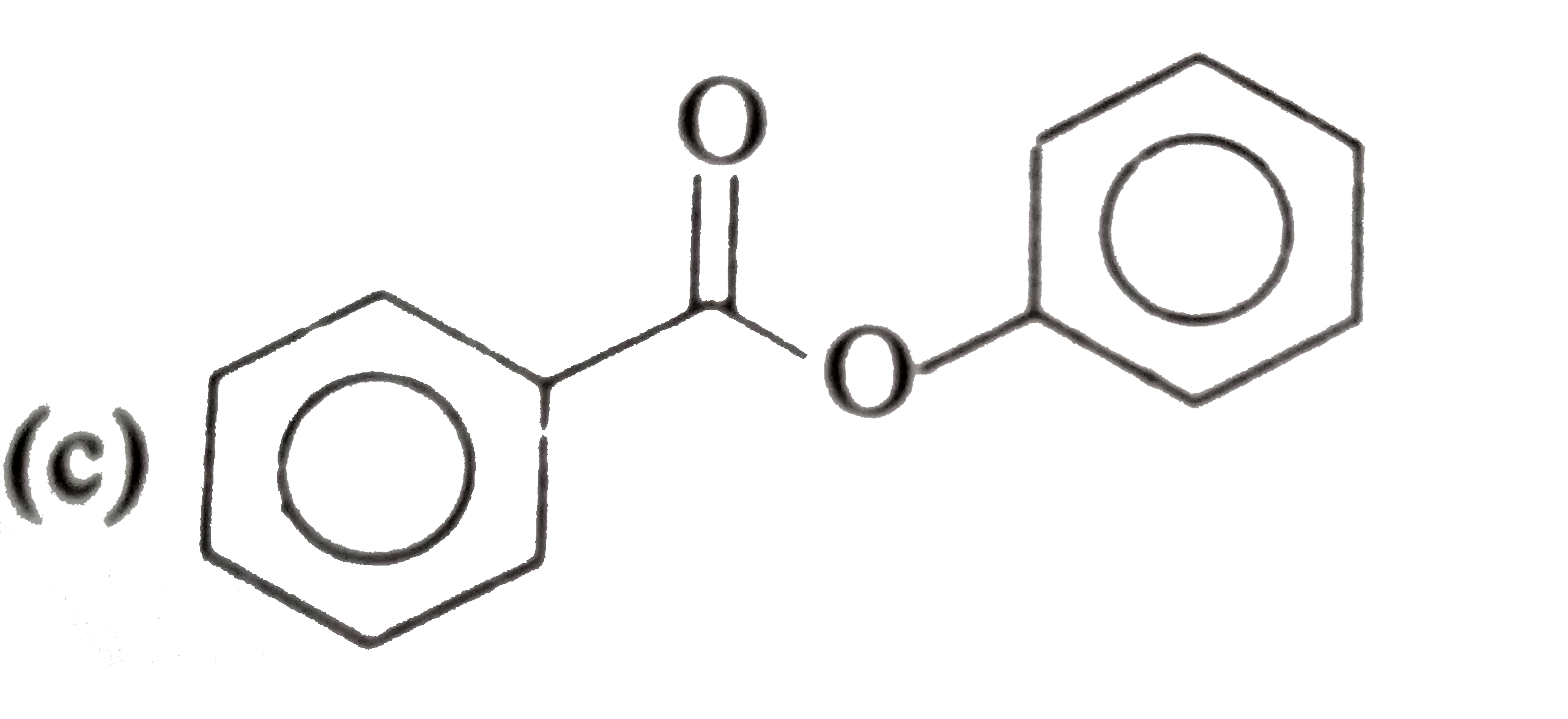 D
D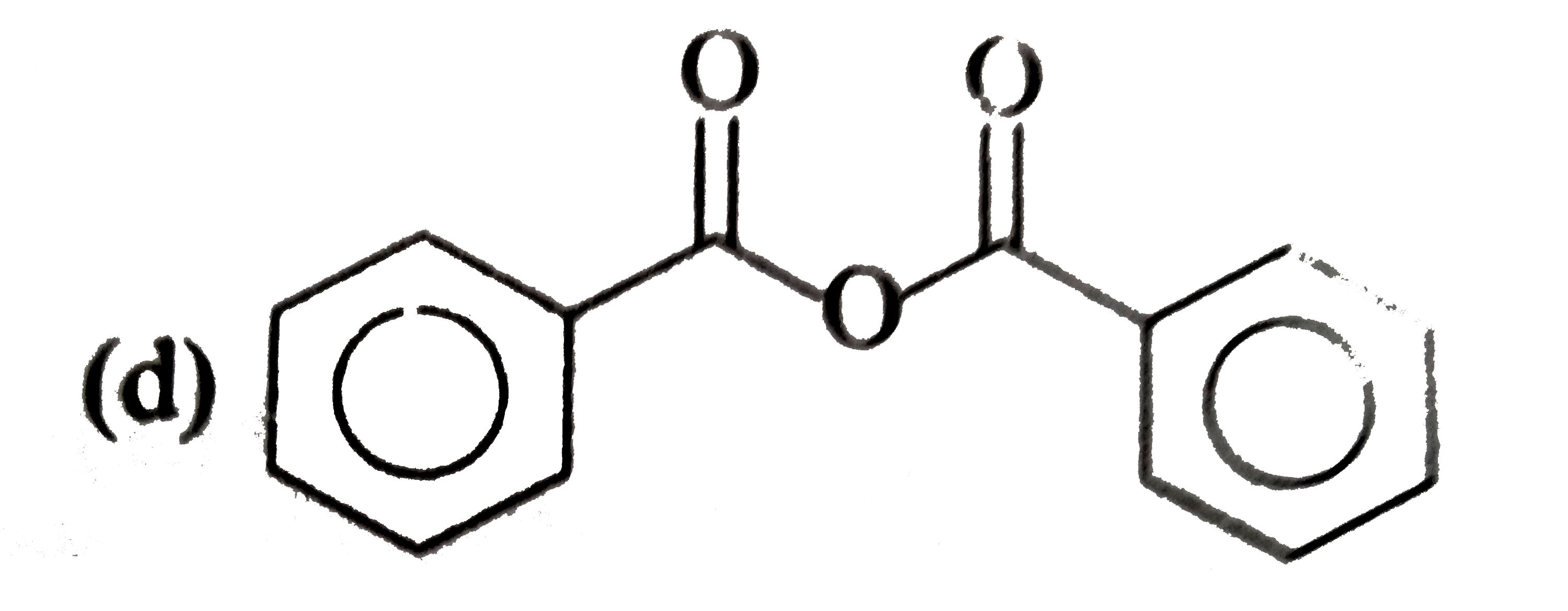
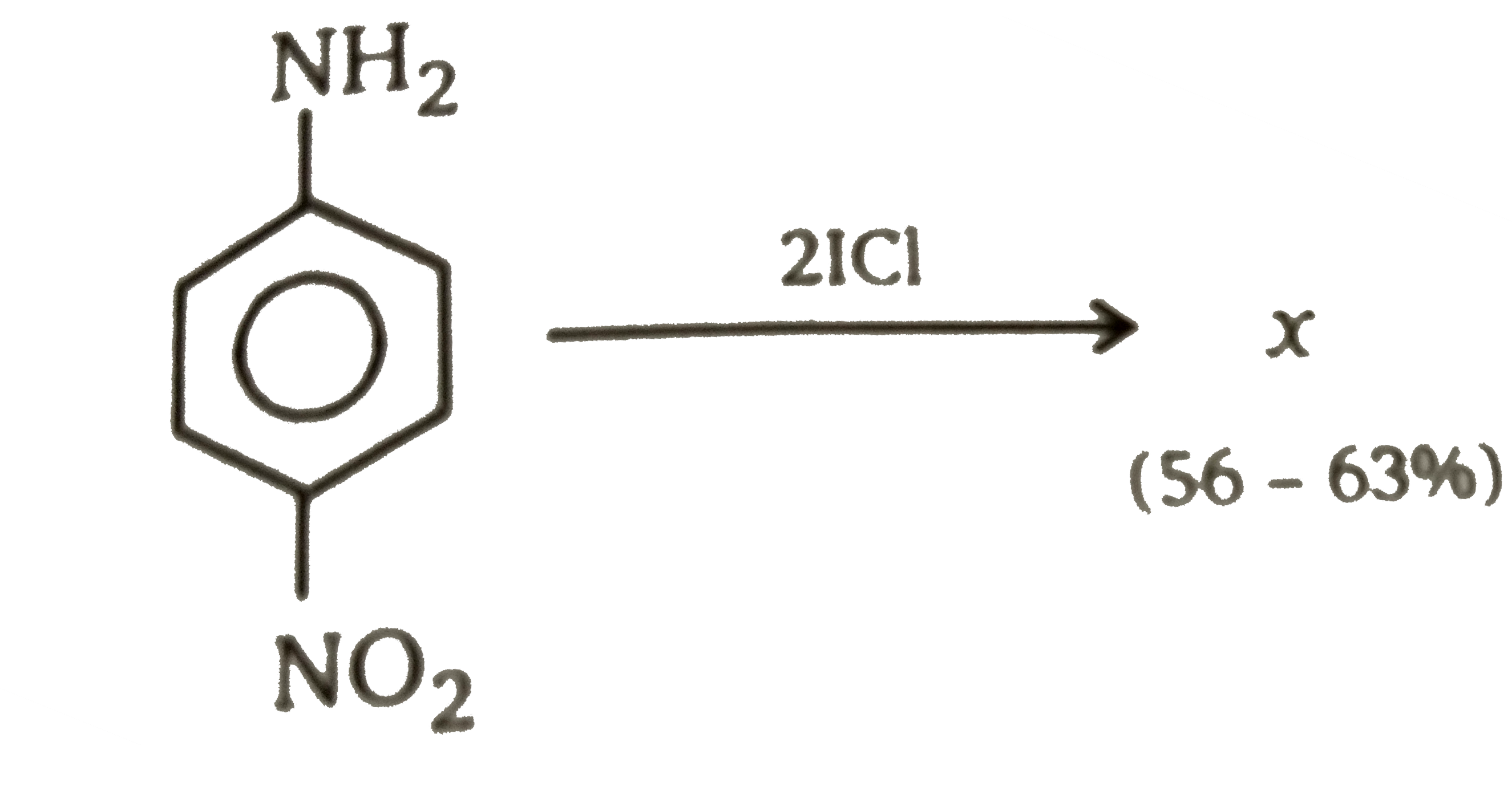 , Major product (x) in this reaction is: A
, Major product (x) in this reaction is: A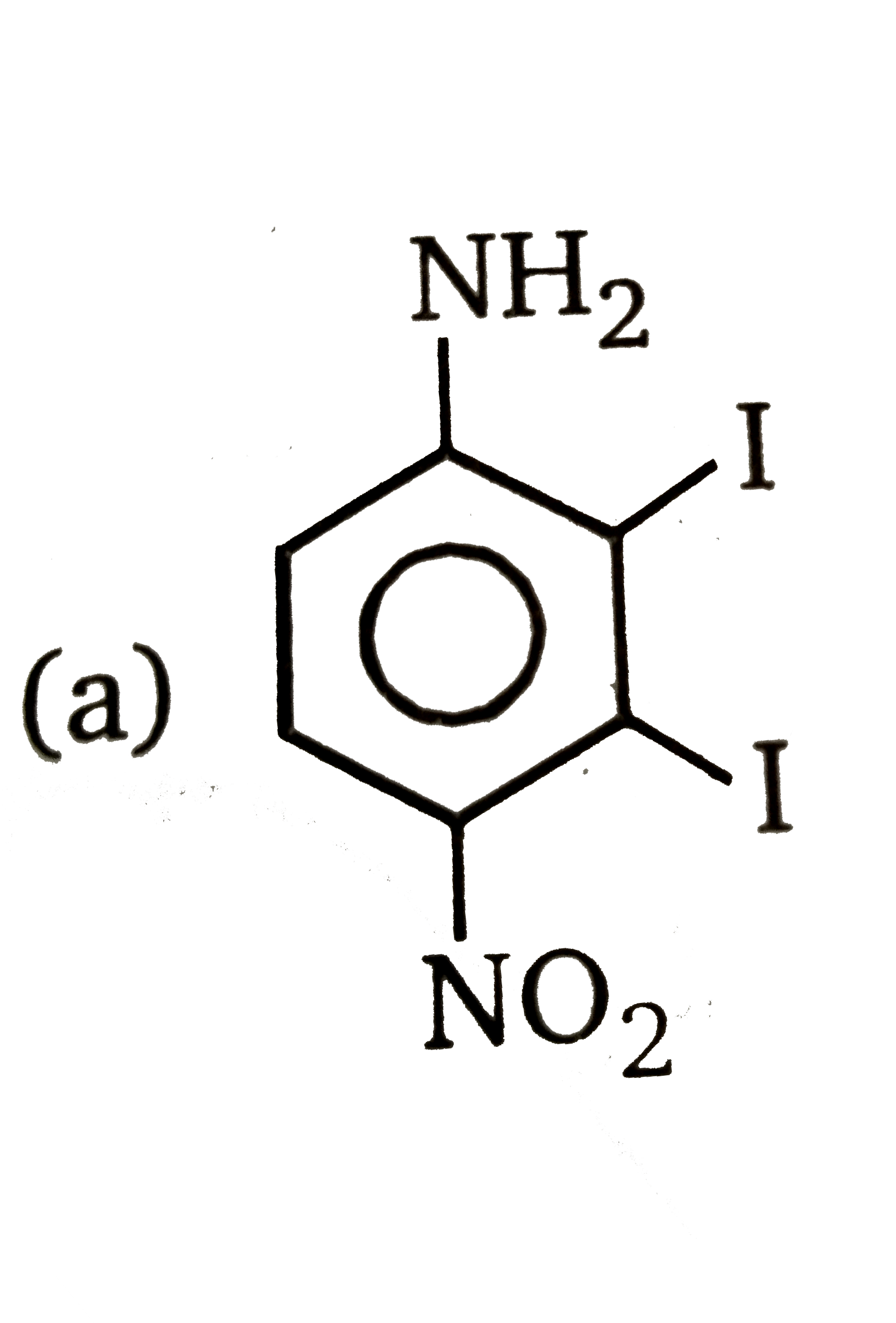 B
B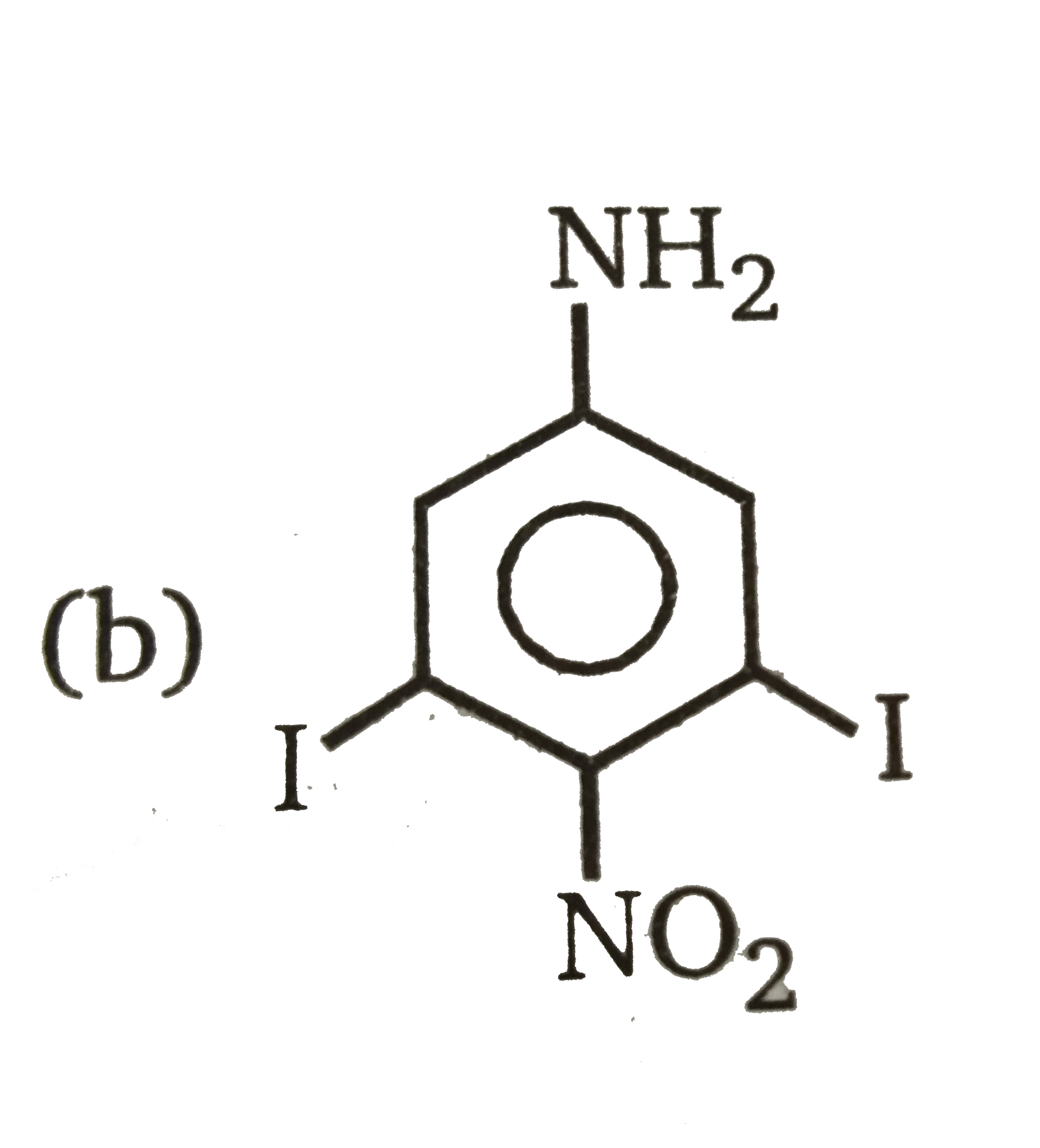 C
C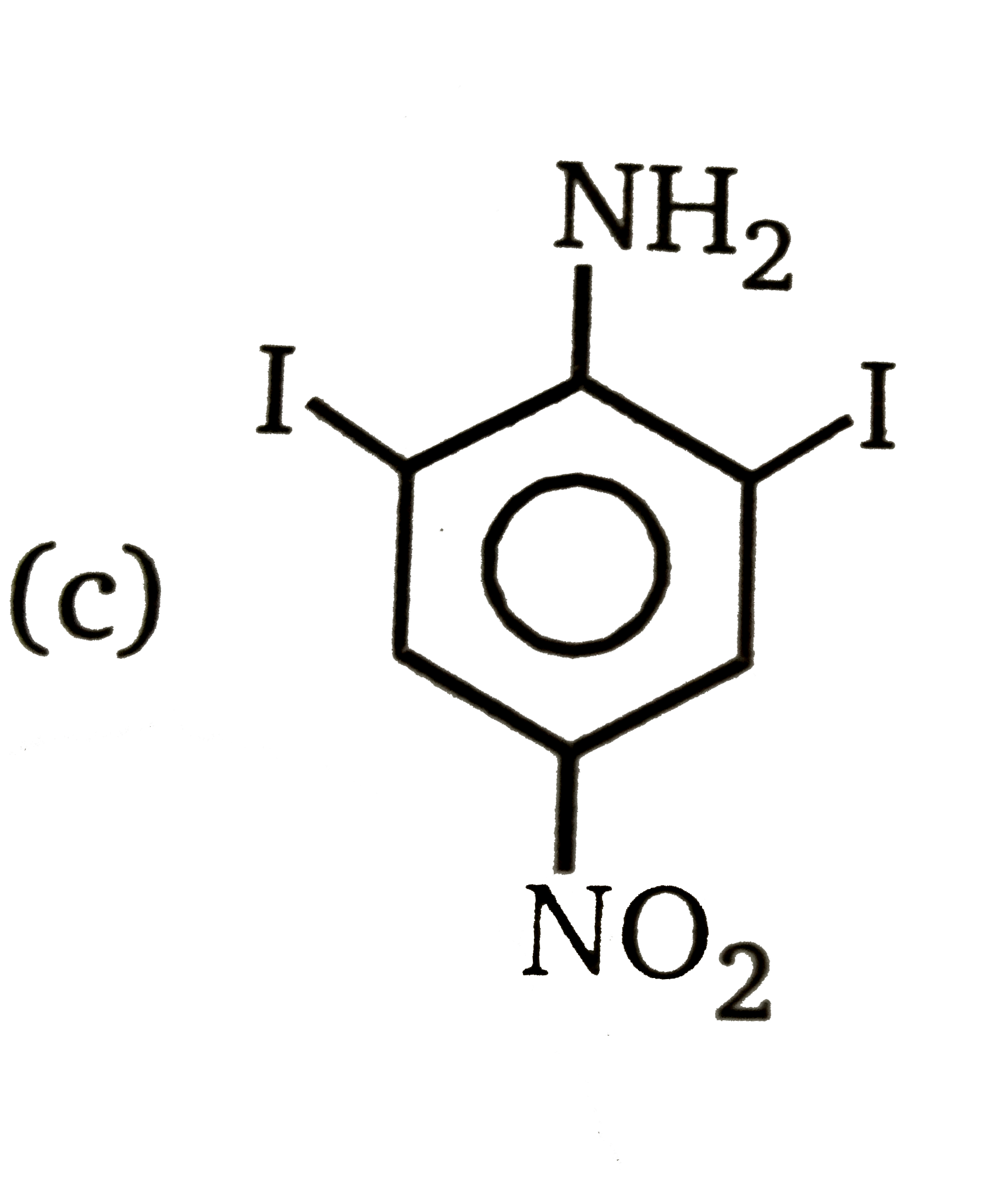 D
D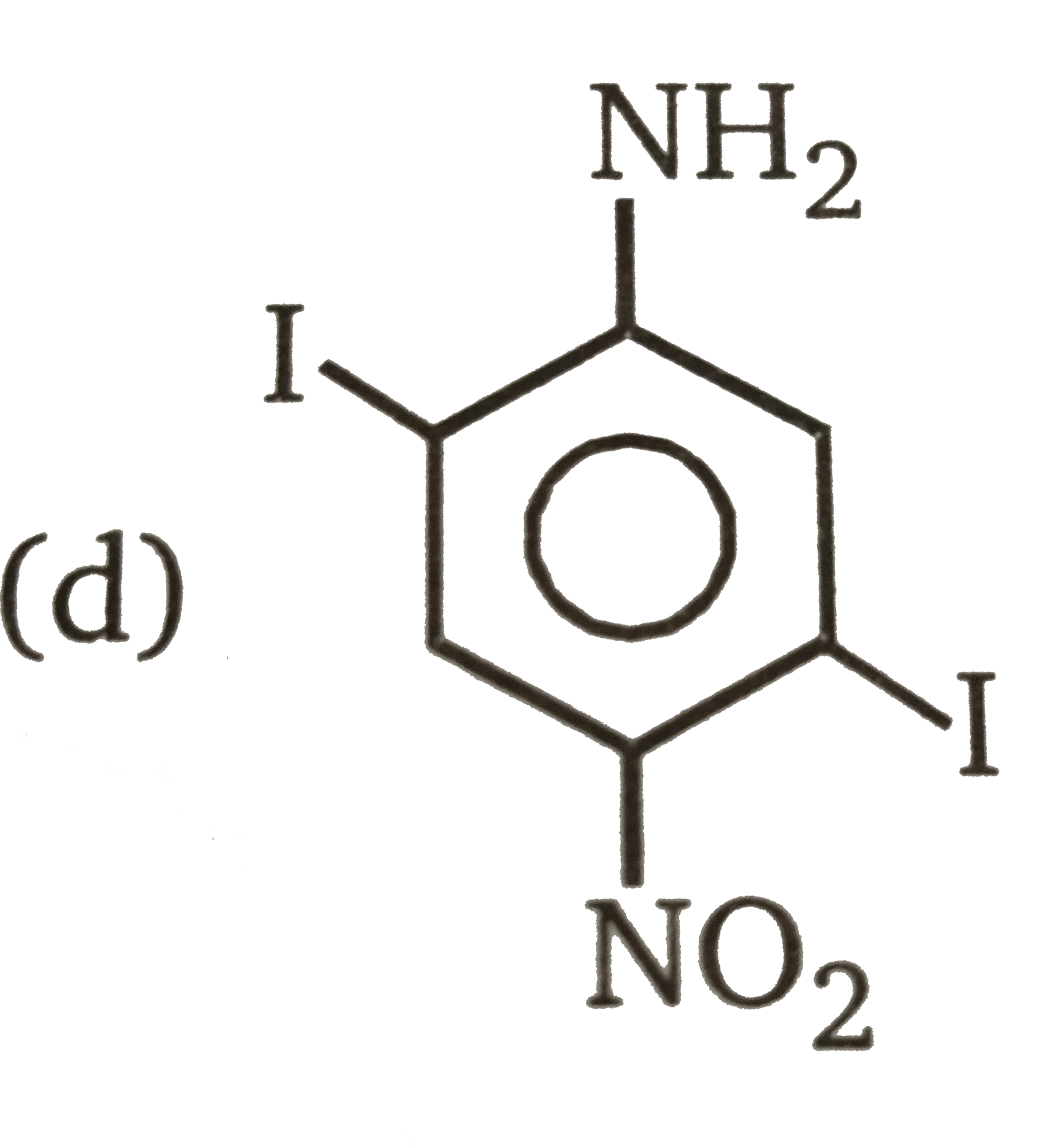
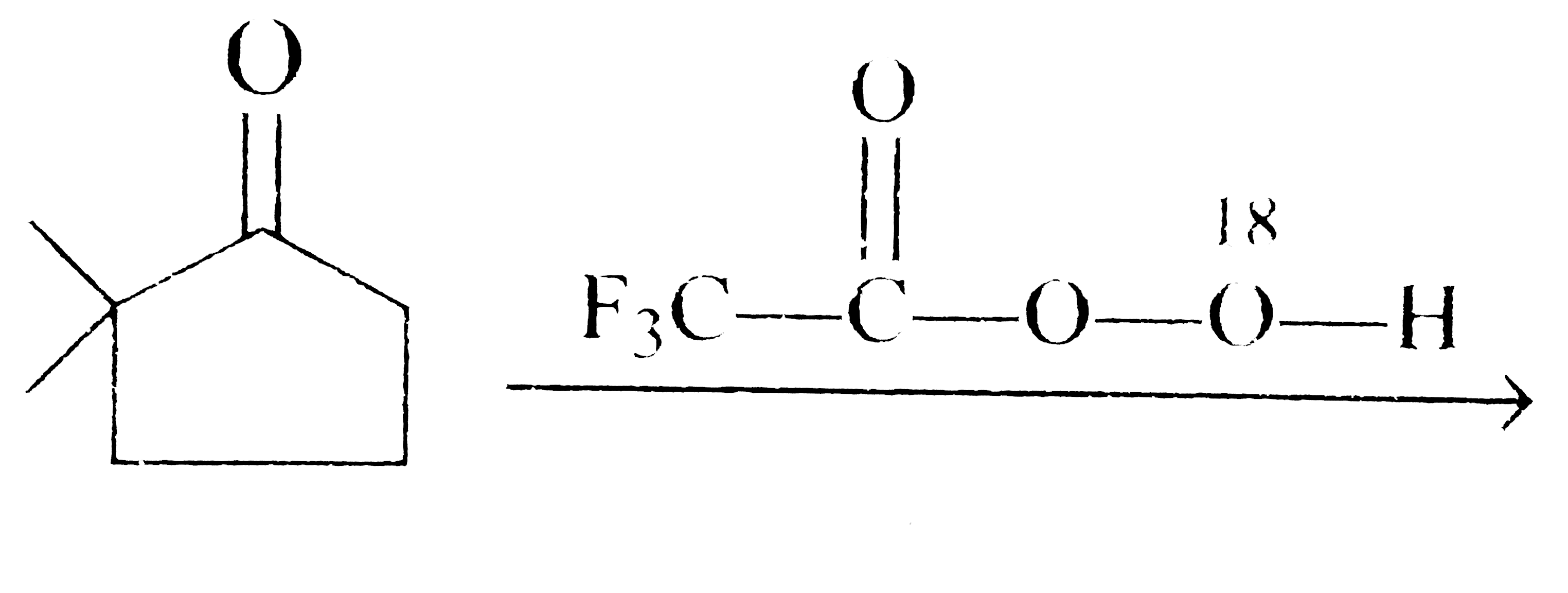 Major product (X) :A
Major product (X) :A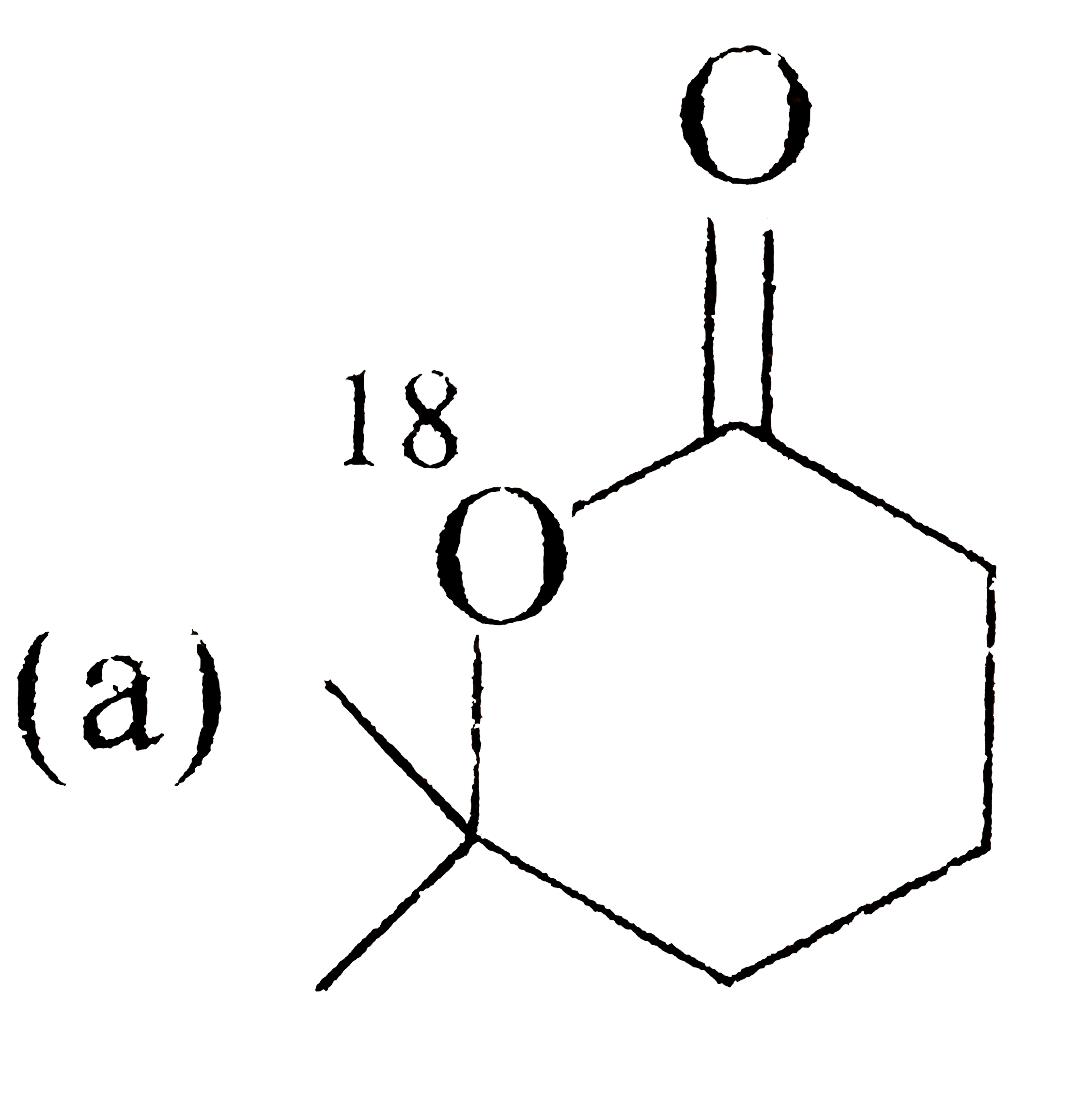 B
B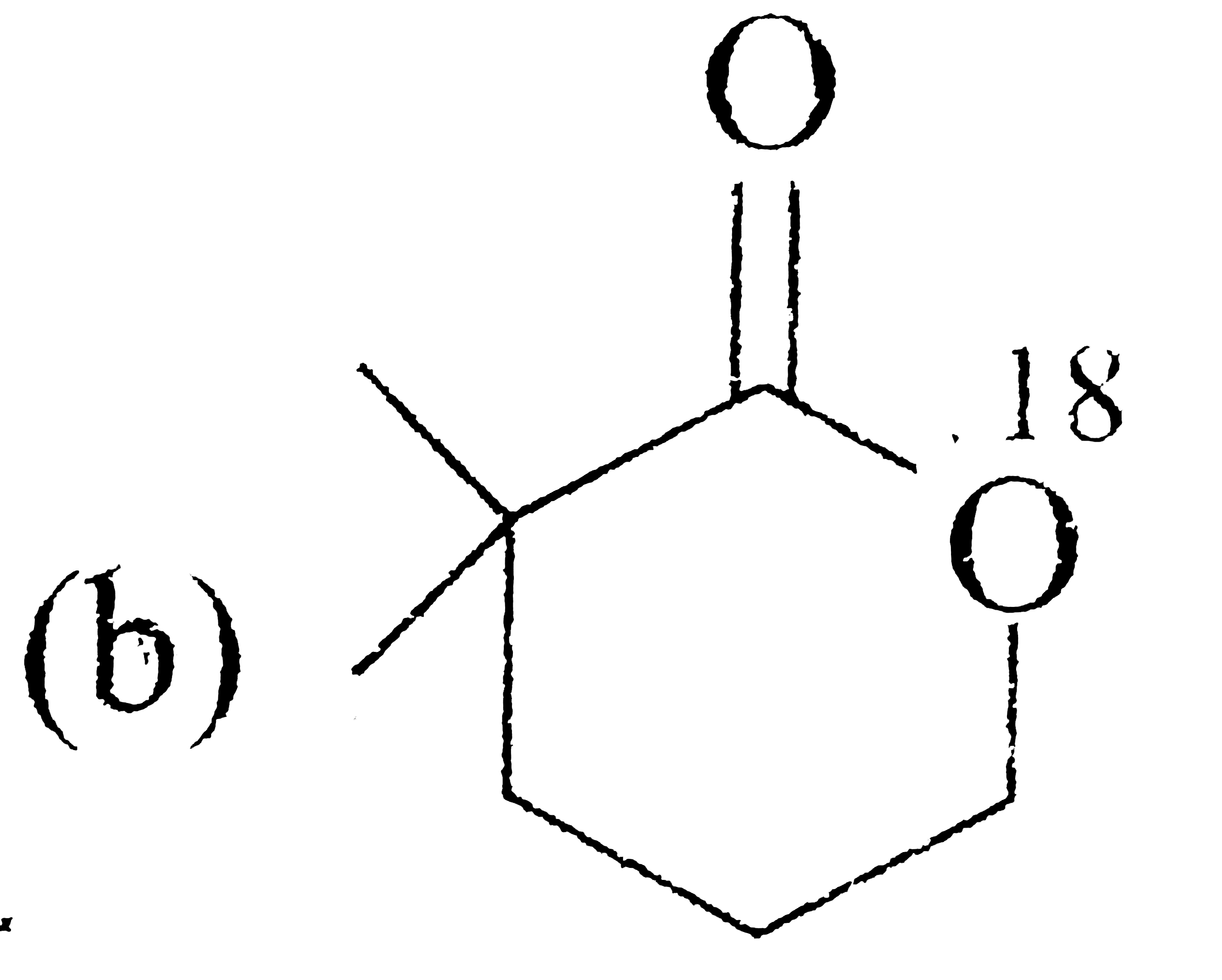 C
C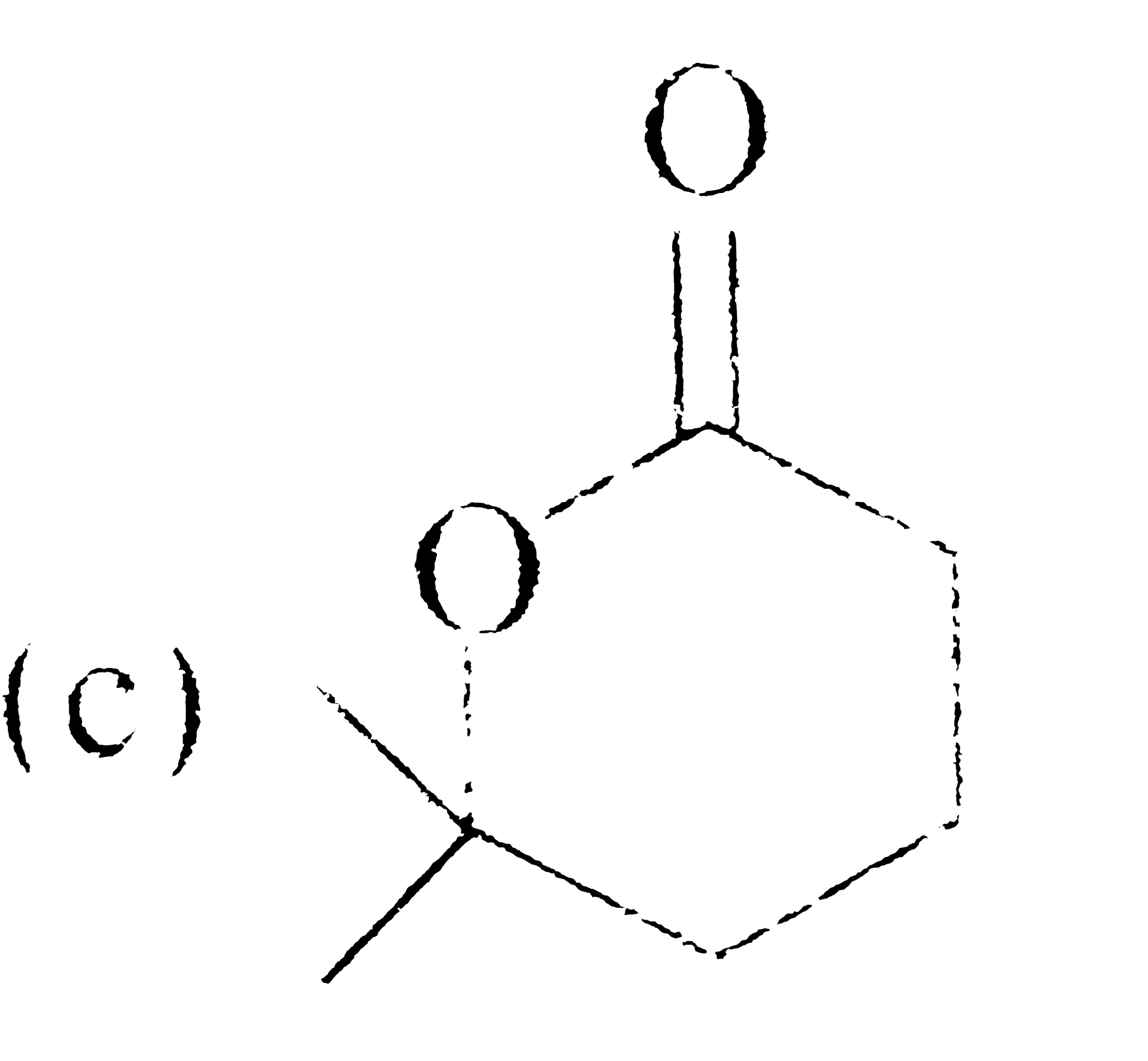 D
D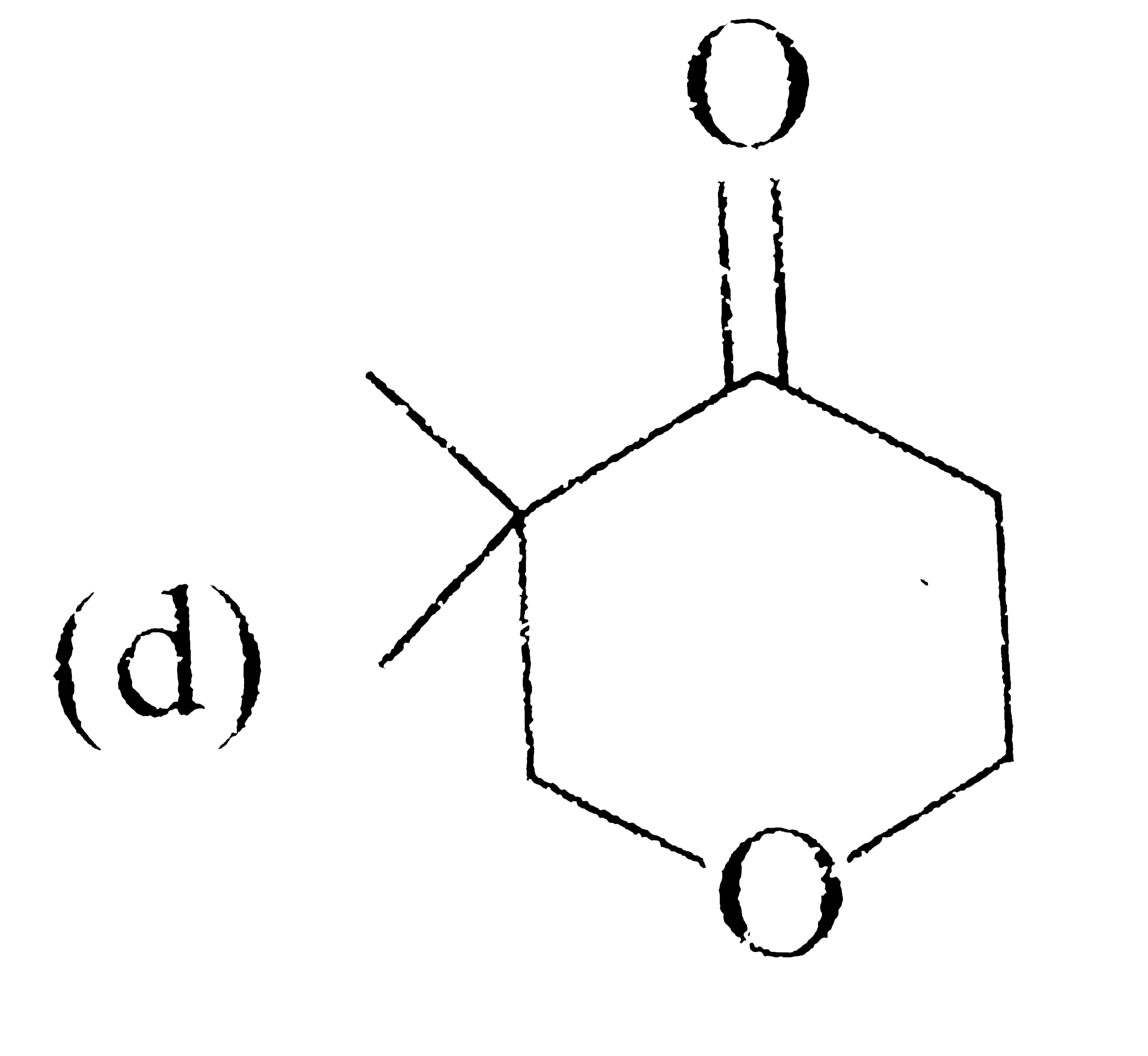


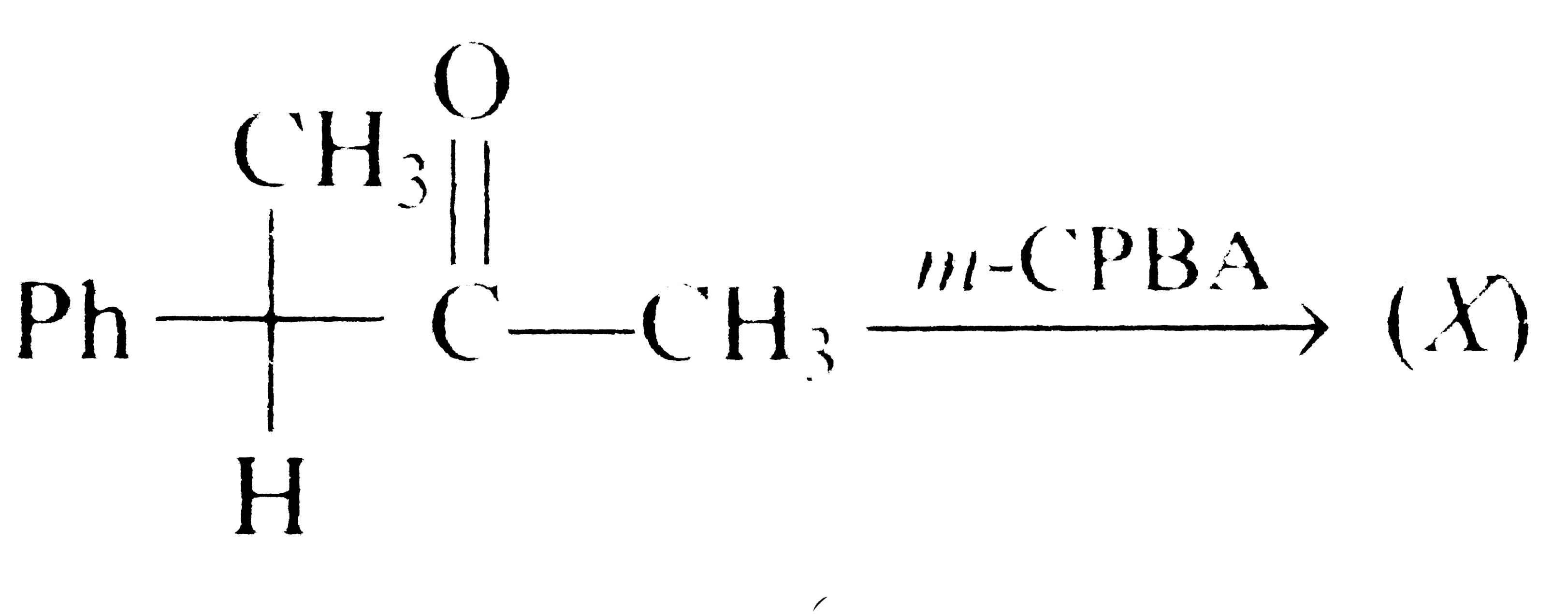 , Find major product (X) :
, Find major product (X) : 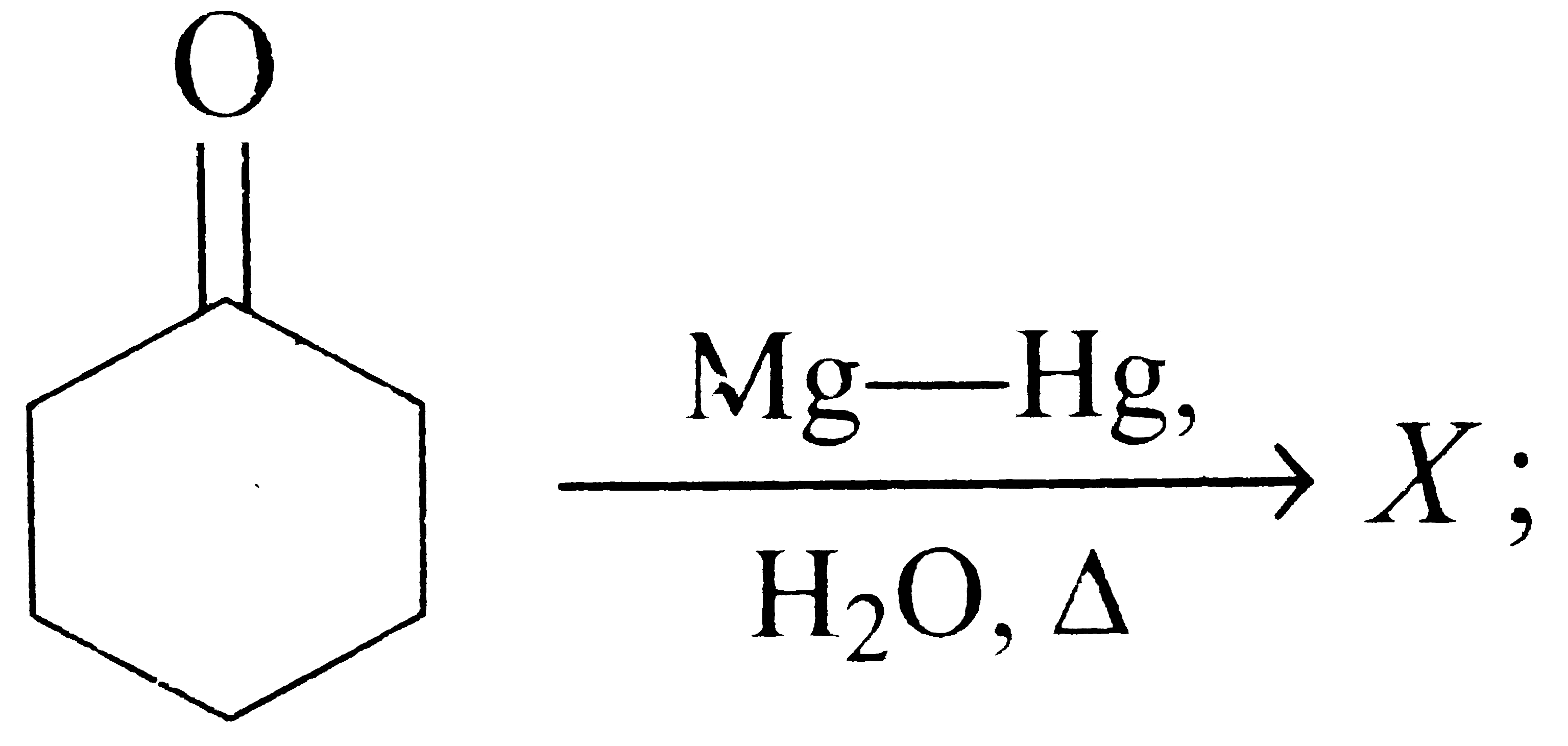 , Find out final product (X) :
, Find out final product (X) :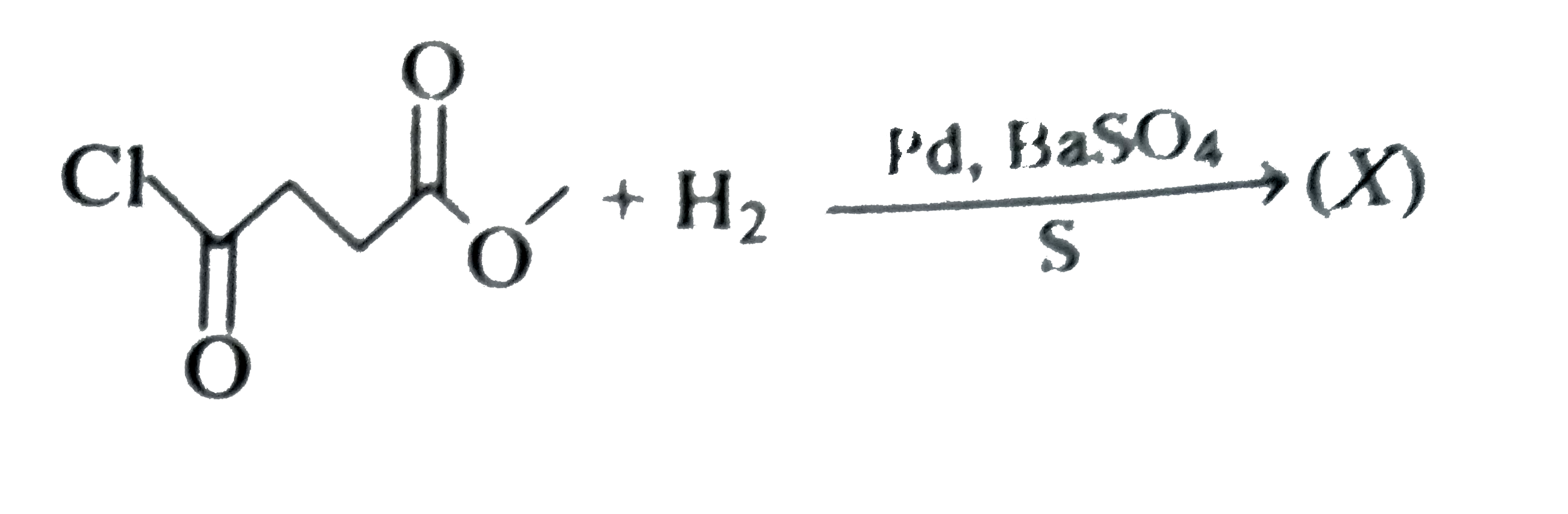
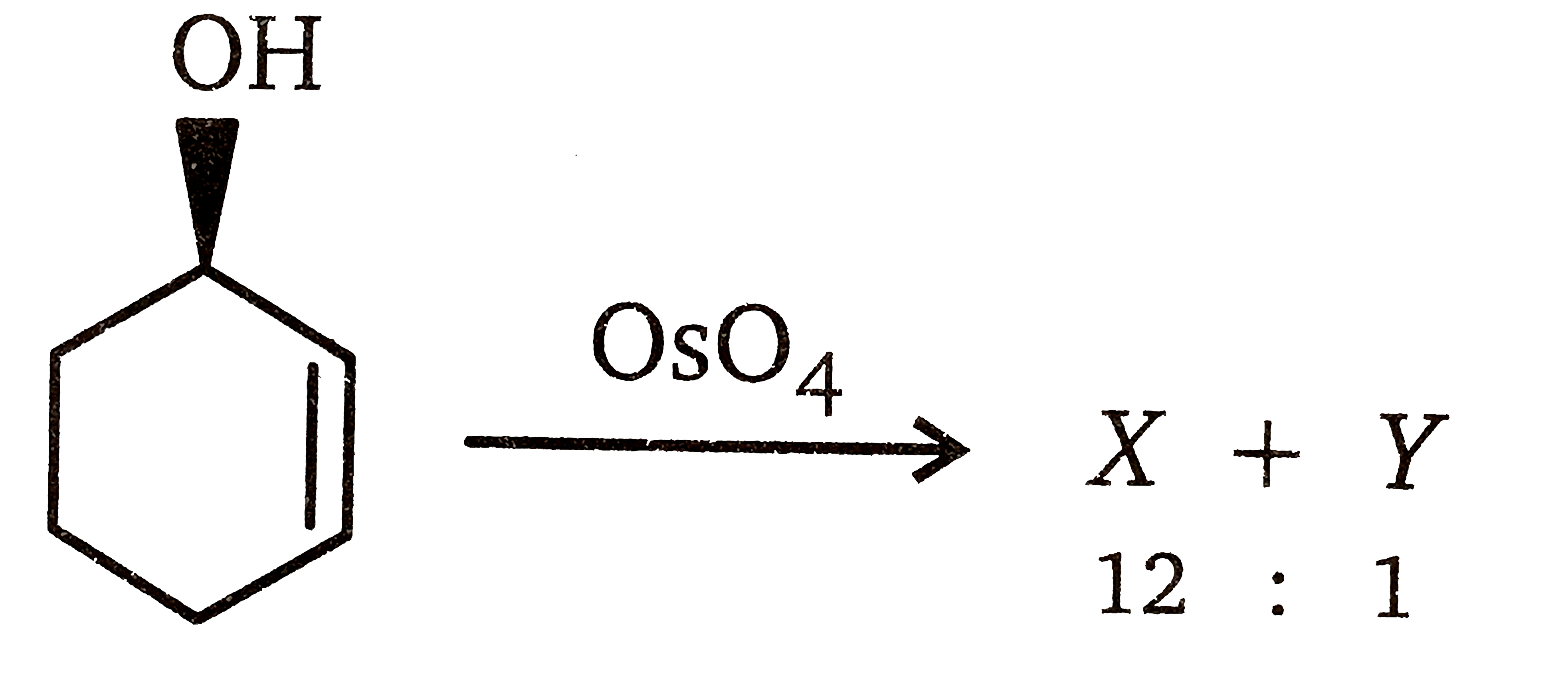 Product (X) will be:
Product (X) will be: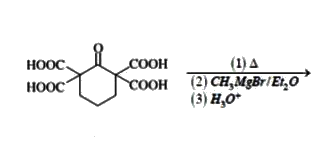 Product (X), X is:
Product (X), X is: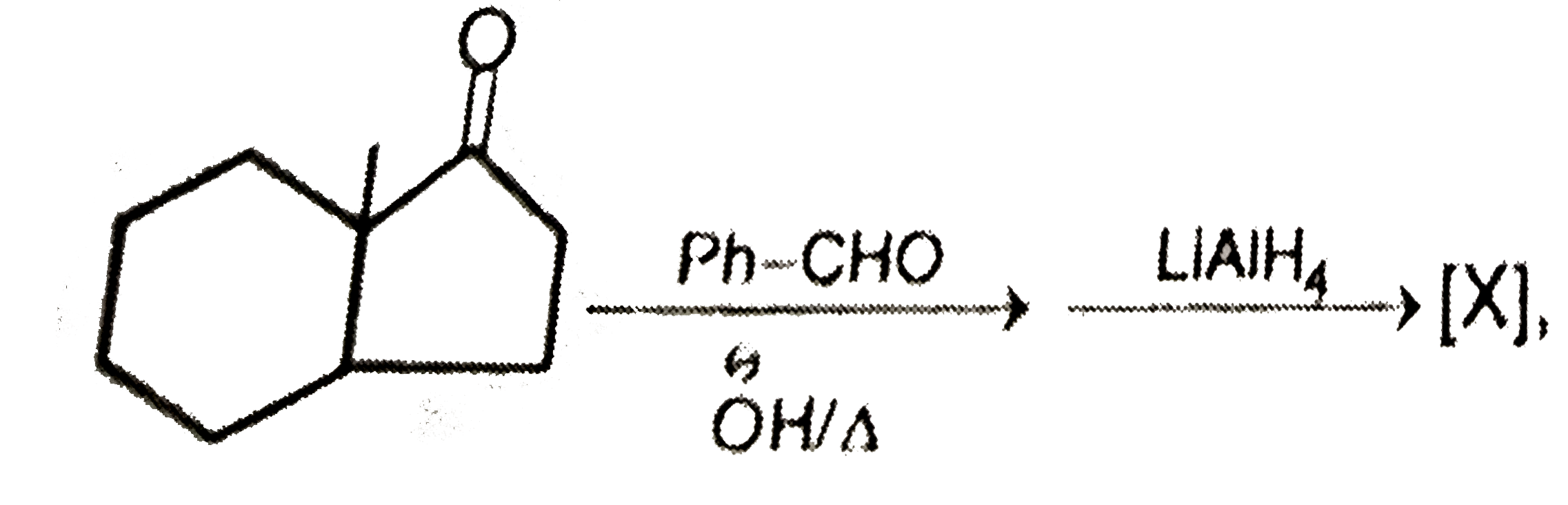 [X], product (X) in this reaction is
[X], product (X) in this reaction is