Which reaction is not feasible?
A
HC≡CH+Li→Li+−C≡−CLi++H2
B
4NaNO3Δ−−→2Na2O2+4NO2+O2
C
LiCl+2H2O→LiCl.2H2O
D
MgCl2+6H2O→MgCl2.6H2O
Text Solution
The correct Answer is:A, B
(a) Li does not form ethynide.
(b) 2NaNO3Δ−−→2NaNO2+O2
|
Similar Questions
Knowledge Check
A reaction is not feasible if
AΔH is positive and ΔS is also positiveBΔH is positive and ΔS is negativeCΔH is negative and ΔS is also negativeDΔH is negative and ΔS is positiveWhich reaction is least feasible
A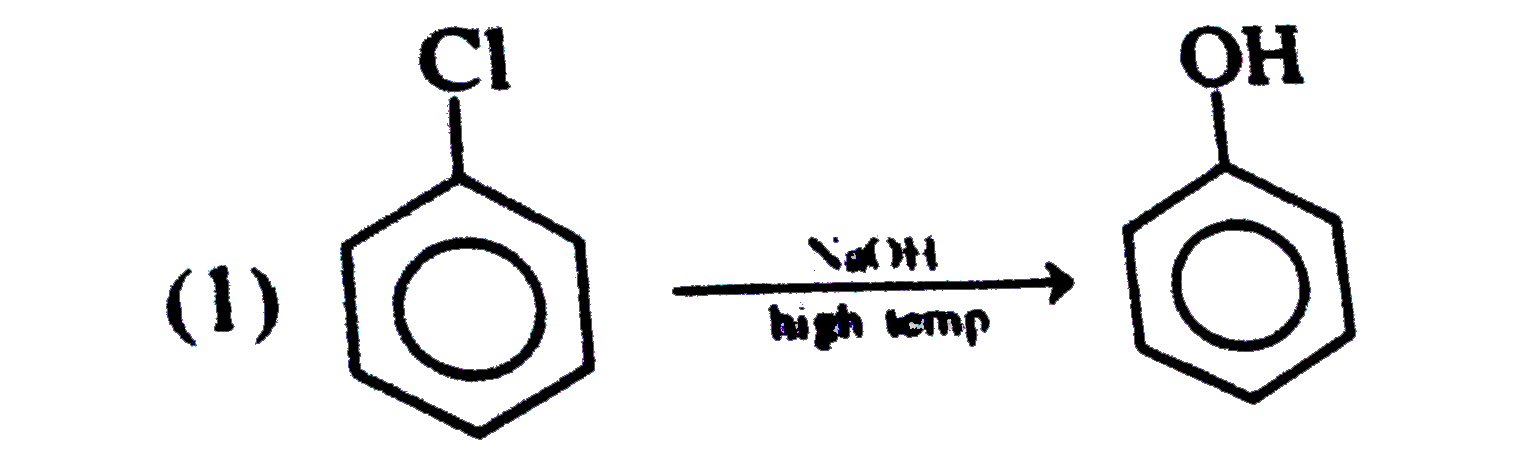 B
B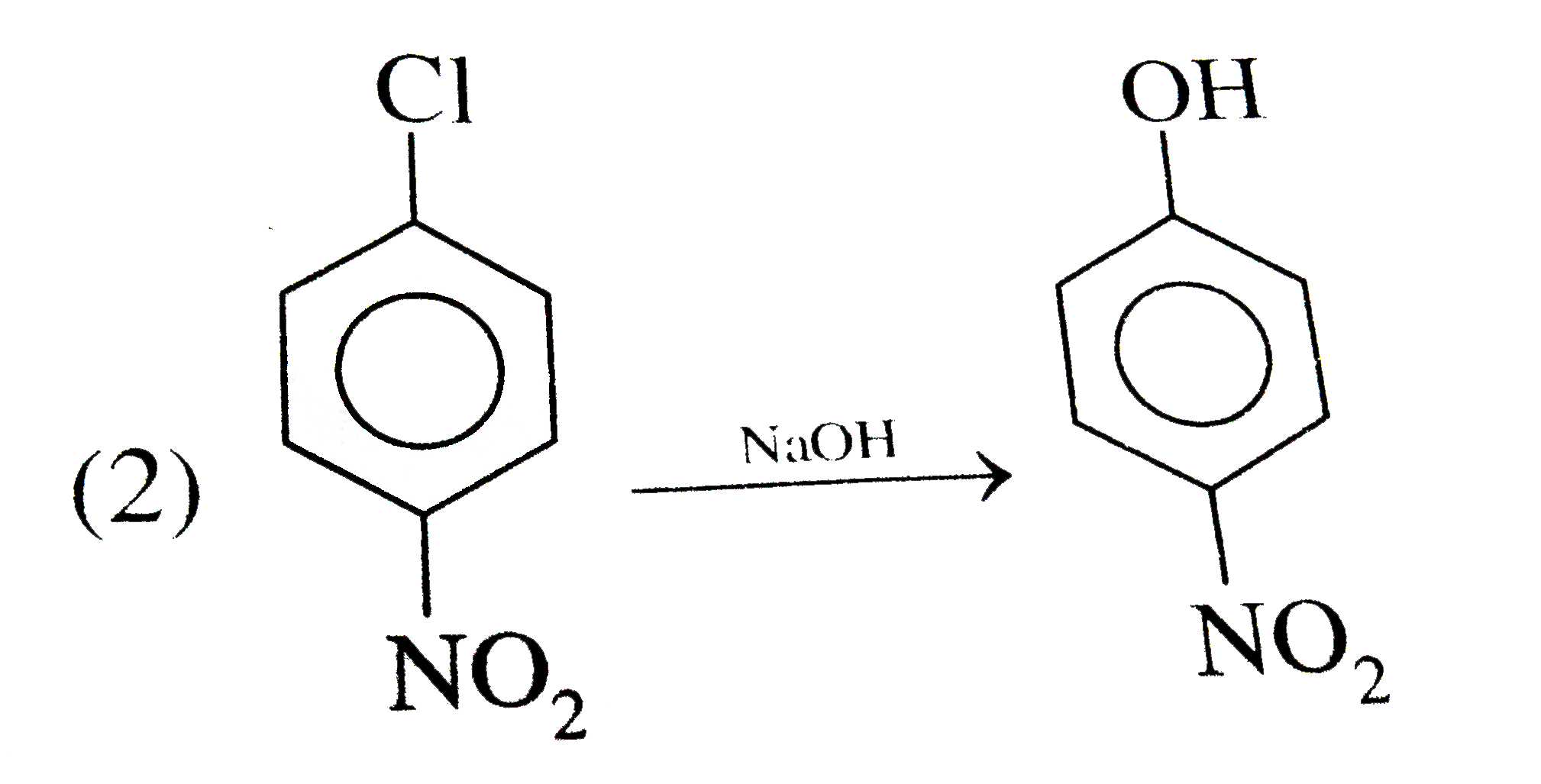 C
C D
D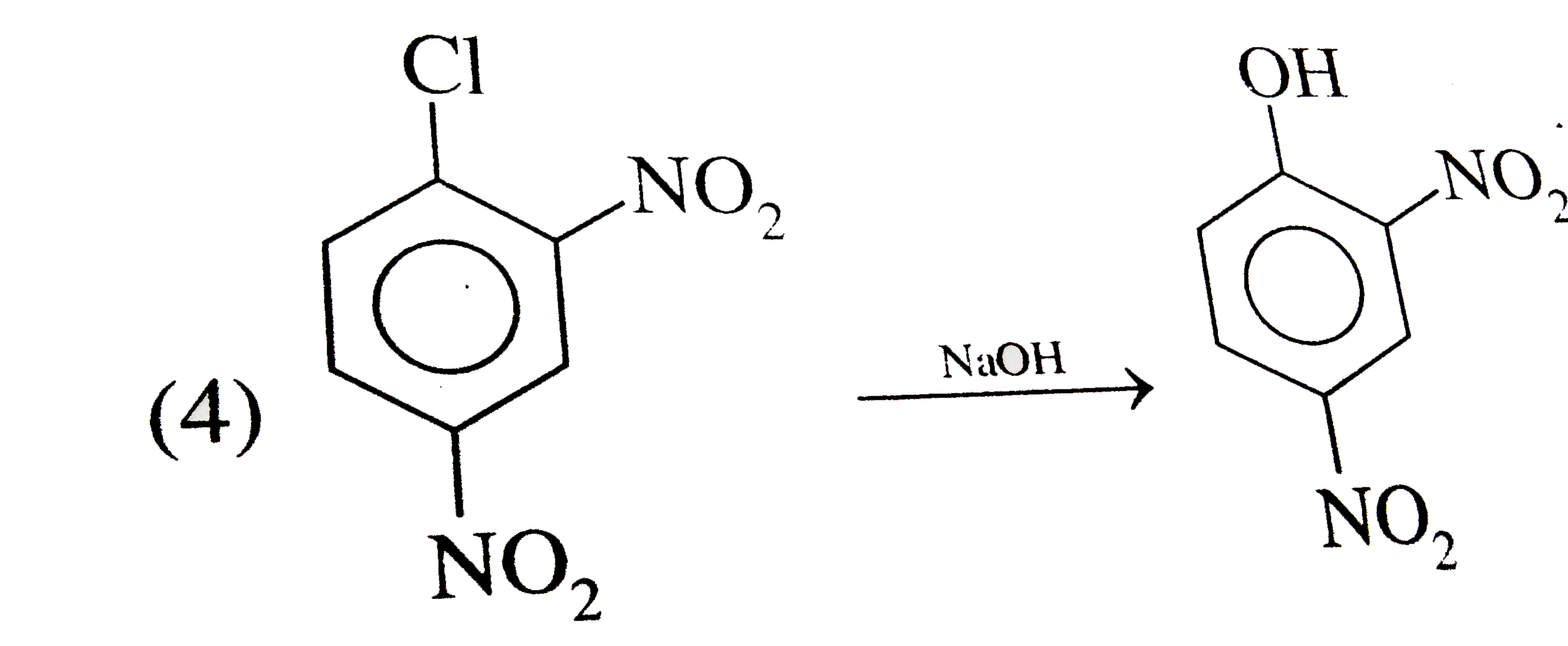
Which of the reaction is not feasible?
AHC≡CNa+CH3OH→BHC≡CH+CH3Li→CHC≡CH+NaH→DHC≡CH+NaCN→
Which of the following reactions is not feasible?
View SolutionWhich of the following reactions is not feasible?
View SolutionWhich of the following reaction is not feasible?
View SolutionWhich of the following reactions is not feasible ?
View SolutionWhich of the following reactions is not feasible ?
View Solution

