Questions given below are based on electronic configurations of the elements. The three elements X,Y and Z with the electronic configuration shown below all form hydrides:
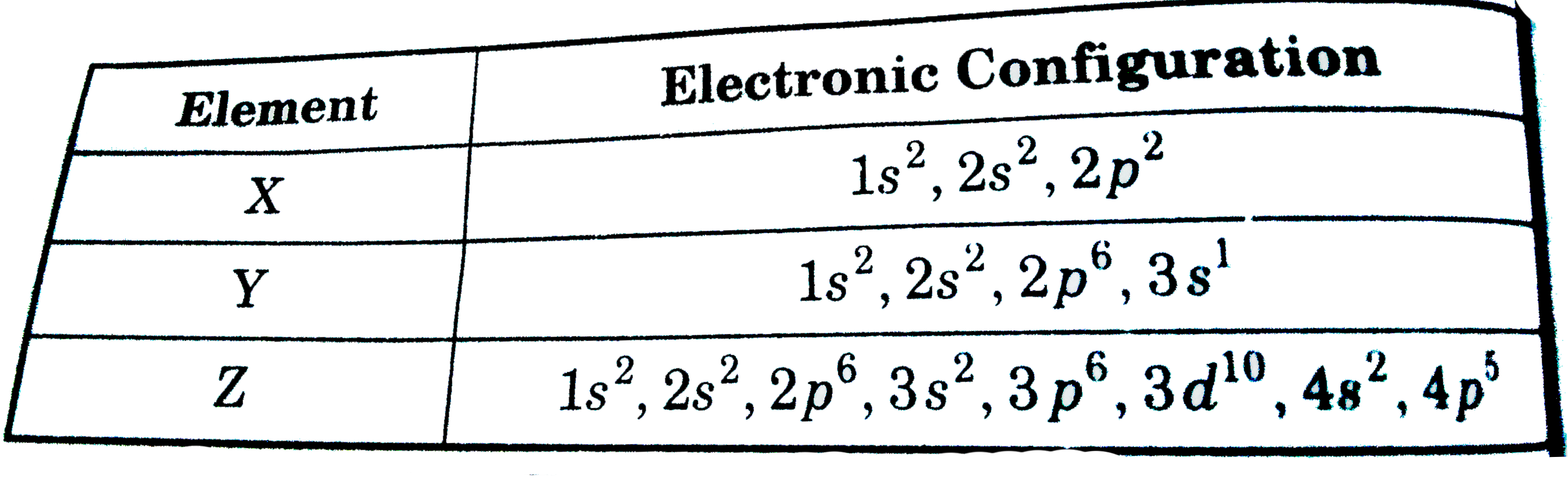
Which line of properties (A,B,C or D) correctly lists properties of the hydrides of there elements?
Hydride of XHydride of YHydride of Z Colourless gas insoluble in H2OSilver/grey solid, react with H2O to form an alkaline solutionColourless gas form a strong acid inH2O
Hydride of XHydride of YHydride of Z Colourless liquid no reaction with H2OSilver/grey solid, form H2O Ionic solid with formula ZH
Hydride of XHydride of YHydride of Z Colourless gas found naturallyDoes not conduct electricity in the molten stateColourless gas reacts with Cl2
Hydride of XHydride of YHydride of ZNon-polar compound reacts with Cl2 in lightSilver/grey ionic solid with formula YH2Form when water is added to phosphrous and element Z
The correct Answer is:A
Step by step video, text & image solution for Questions given below are based on electronic configurations of the elements. The three elements X,Y and Z with the electronic configuration shown below all form hydrides: Which line of properties (A,B,C or D) correctly lists properties of the hydrides of there elements? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
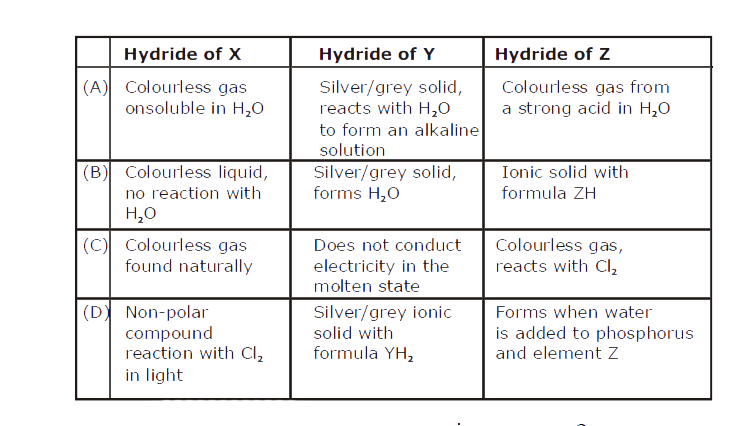
Question given below are based on electronic configuration of the element. The three X,Y and Z with the electronic configurations shown below all formhydrides:
Element Electronic configuration
X 1s2,2s2,2p3
Y 1s2,2s2,2p6,3s1
Z 1s2,2s2,2p6,3s2,3d10,4s2,4p5
Whcih of the properties (A,B,C or D) correctly list properties of the hydrides of these elements
View SolutionThe electronic configuration of four elements A, B, C and D are:
View Solution
Knowledge Check
Questions given below are based on electronic configurations of the elements. The three elements X,Y and Z with the electronic configuration shown below all form hydrides:
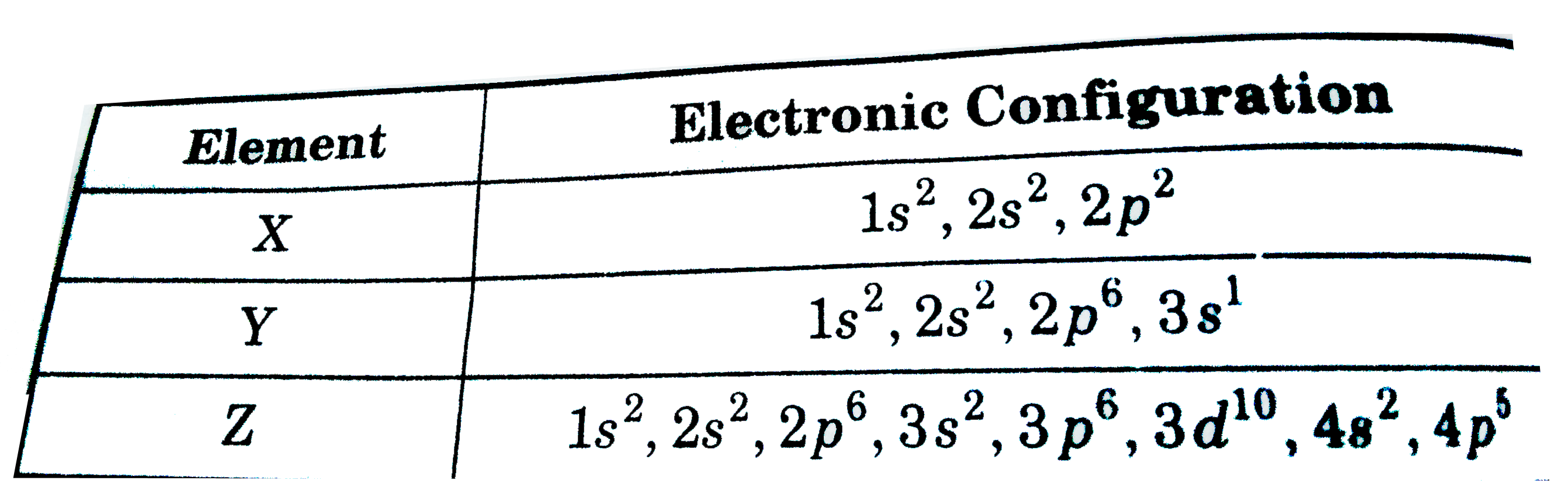
Which of the following exists as gas ?AX2BY2CZ2DAll of theseThe element which readily forms an ionic bond has the electronic configuration
A1s22s22p3B1s22s22p1C1s22s22p2D1s22s22p63s1Question given below are based on electronic configuration of the element. The three X,Y and Z with the electronic configurations shown below all formhydrides:
Element Electronic configuration
X 1s2,2s2,2p3
Y 1s2,2s2,2p6,3s1
Z 1s2,2s2,2p6,3s2,3p6,3d10,4s2,4p5
Which of the following exists as gas?AX2BY2CZ2DAll of the above
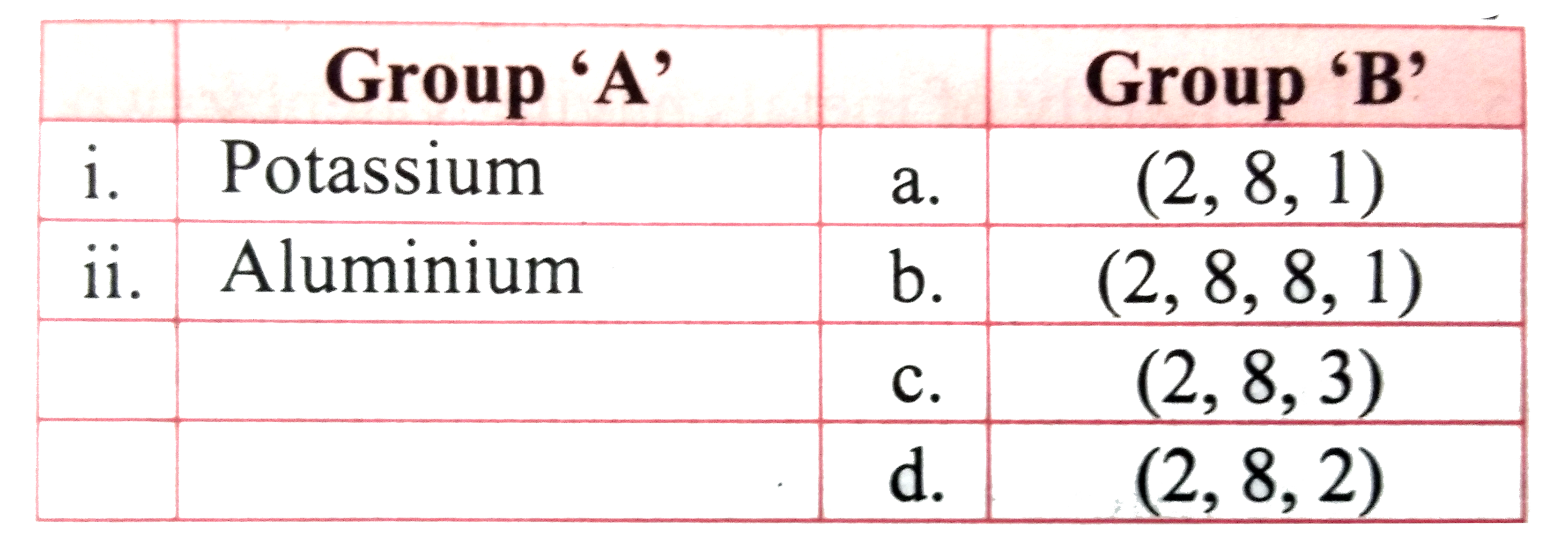
Match the elements given in Group 'A' with their electronic configuration given in Group 'B'.
View SolutionElectronic configuration of four elements A,B,C and D are given below
View SolutionElectronic configurations of four elements A, B, C and D are given below
View SolutionElectronic configuration of few elements are given below. Mark the incorrect match.
View SolutionElectron configurations of few elements are given below,Mark the incorrect match.
View Solution
