defined the following terms. (i) Isotherm (ii) Critical Temperature (iii) Critical Pressure (iv) Critical VolumeSolution in Tamil
(i) Isotherm :
At any constant temperature when pressure and wice versa. Such P-V curves at constant temperature are known as isotherms. (ii) Critical Temperature (TC)
It is defined as the charactersitic temperature of a gas above which no liquefaction occurs.
(iii) Critical Pressure :(PC)
It is defined as the minimum pressure required to liquefy 1 mole of a gas present at its critical temperature.
(iv) Ctritical volume: (VC)
The volume occupied by 1 mole of gas ideal behaviour is measured in termsof a ratio of PV to nRT thsi is termed as compressibility factor.
Z=PVnRT
Similar Questions
Define the following terms. Critical Temperature (T_c)
View SolutionDefine the following terms. Critical Pressure (P_c)
View SolutionDefine the following terms. Critical Volume (V_c)
View SolutionWhat is Boyl's temperature? Define Critical temperature and pressure .
View SolutionDefine critical pressure and critical volume
View SolutionBoyle's Temperature || Critical Phenomena-Liquefaction OF Gases || Critical Temperature || Critical Pressure and Critical Volume
View SolutionLiquification OF Gases||Andrew’s Isothermal||Critical Constants and Critical Condition||Boyle’s Temperature
View SolutionConsider the following statements. If the van der Waals parameters of two gases are given as
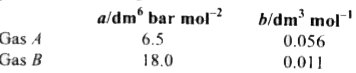
1. Critical volume of A > Critical volume of B
2. Critical pressure of A > Critical pressure of B
3. Critical temperature of A > Critical temperature of B
Which of the above statements are correct?
View Solution

