The condition for standard free energy is ………..Solution in Tamil
298 K , 1 atm
273 K , 1 atm
298∘C,5 atm
25 K, 1 atm
The correct Answer is:A
Step by step video, text & image solution for The condition for standard free energy is ……….. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
गिब्स मानक मुक्त ऊर्जा समीकरण लिखिए।
View SolutionWhat is the condition spontaneity in terms of free energy change?
View SolutionMatch standard free energy of the reaction with the corresponding equilibrium constant
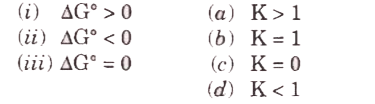
View SolutionThe relationship between standard free energy change and equilibrium constant is expressed as ……………… .
View SolutionThe equilibrium constant of a reaction is 73. Calculate standard free energy change.
View SolutionStatement -1: The Standard free energy changes of all spontaneously occurring reactions are negative .
Statement-2: The standard free energies of the elements in their standard states at 1 bar and 2985K aare taken aszero.
View SolutionThe equilibrium constant of a reaction is 73. Calculate standard free energy change.
View SolutionMatch standard free energy of the reaction with the corresponding equilibrium constant
View Solution

