In each of the following pairs of compounds, Identify the one with more covalent nature and why ?
FeCl2 and FeCl3
Text Solution
According to Fajan's rule,
FeCl3 is more covalent than FeCl2 .
If the oxidation state of the cation is higher, the polarization of anion will be more.
Thus more will be covalent nature in the bonding of the molecule.
|
Similar Questions
Knowledge Check
Which of the following compounds does not give purple colour with FeCl3 ?
A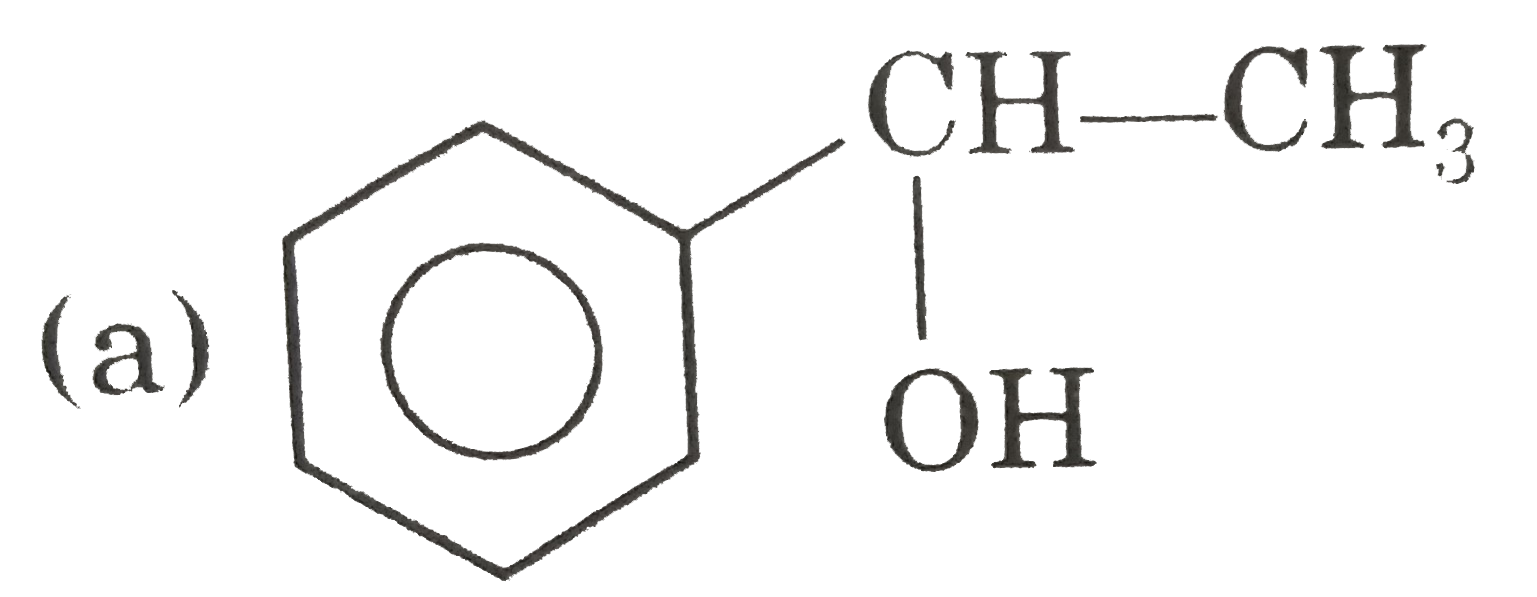 B
B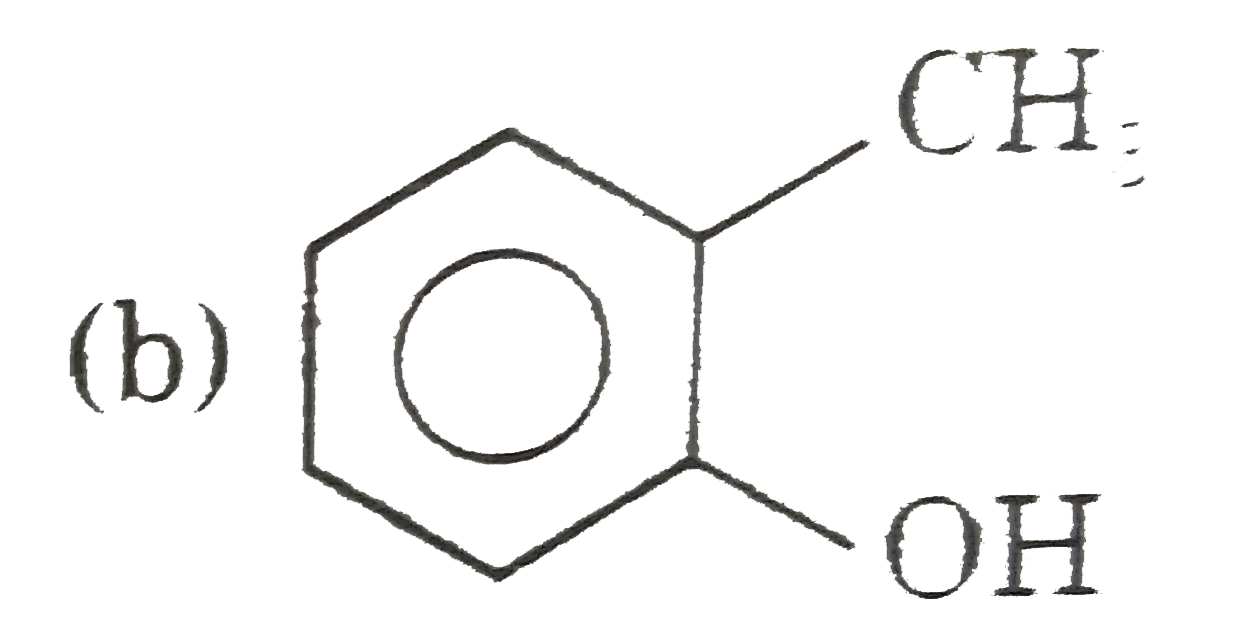 C
C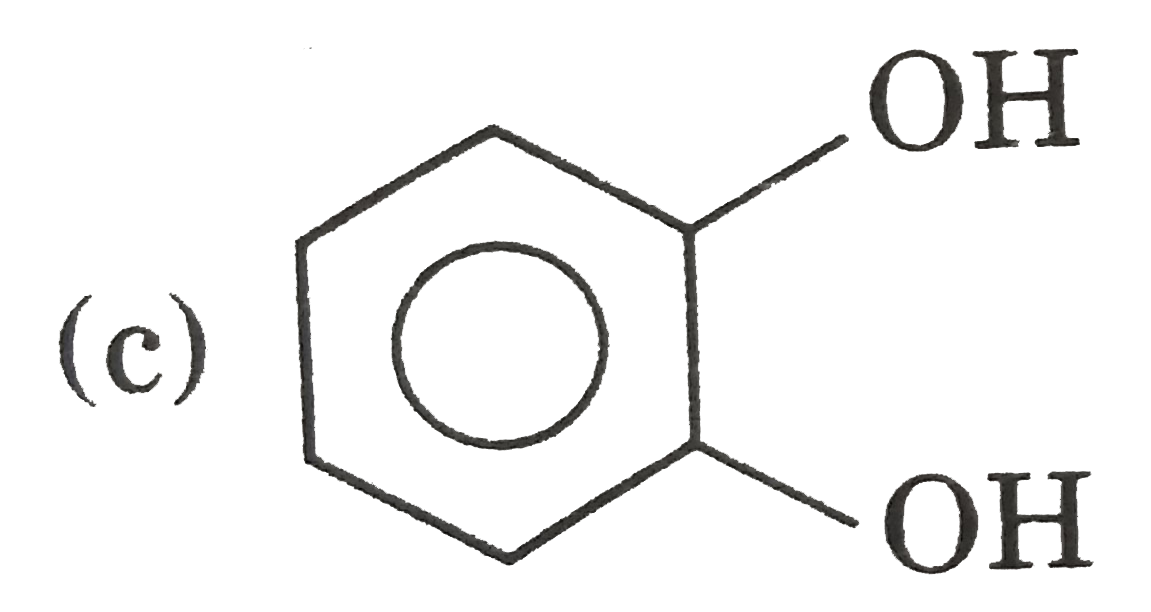 D
D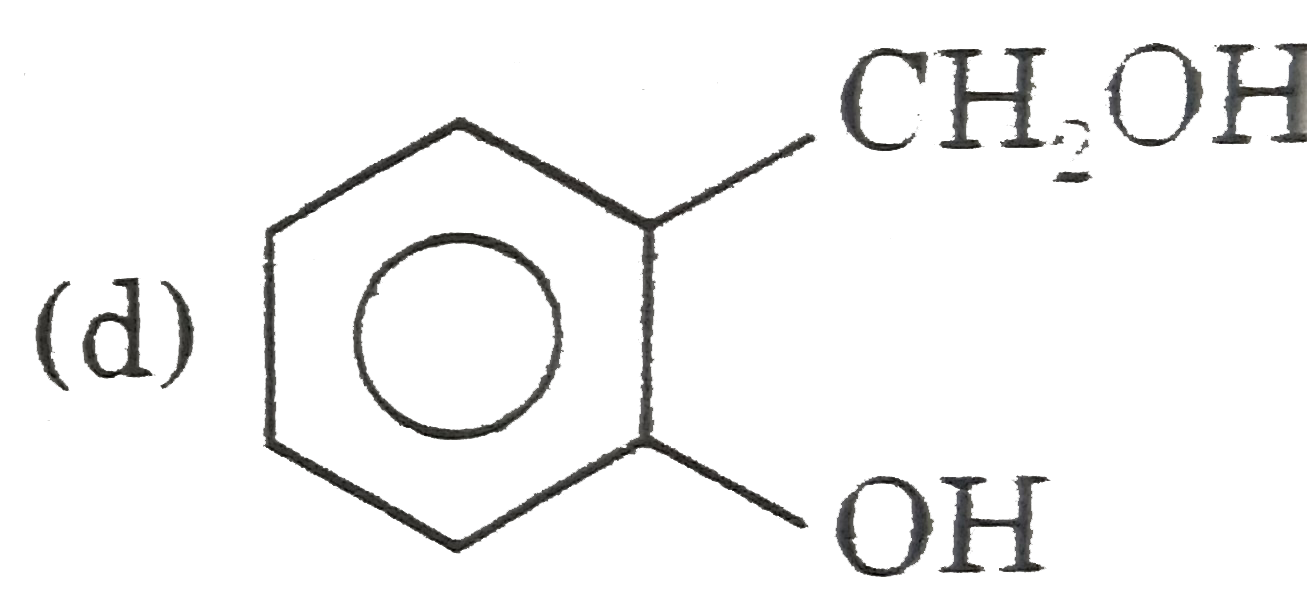
Which of the following compounds will give blue colour when it is converted into Lassaige's extrect and FeCl3?
A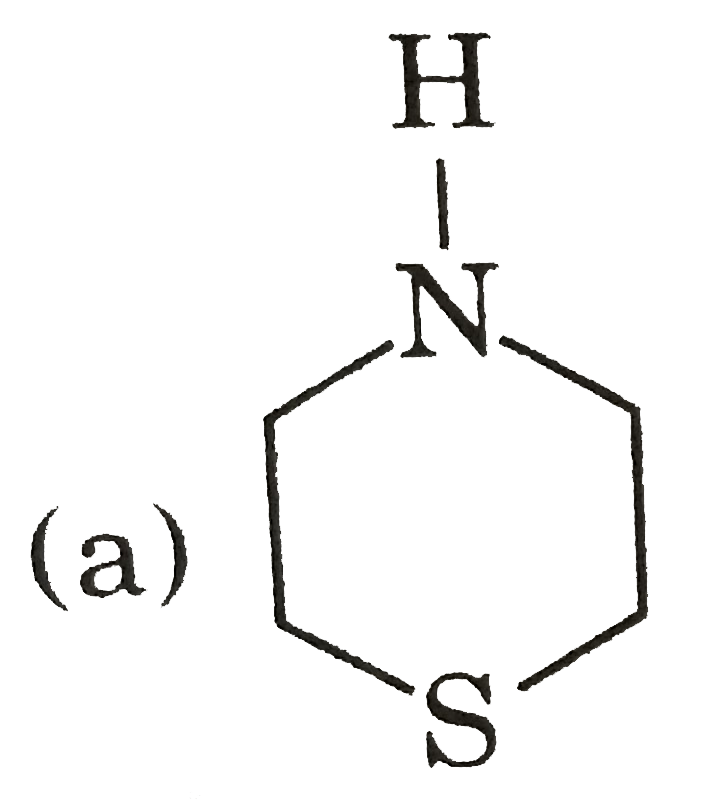 B
B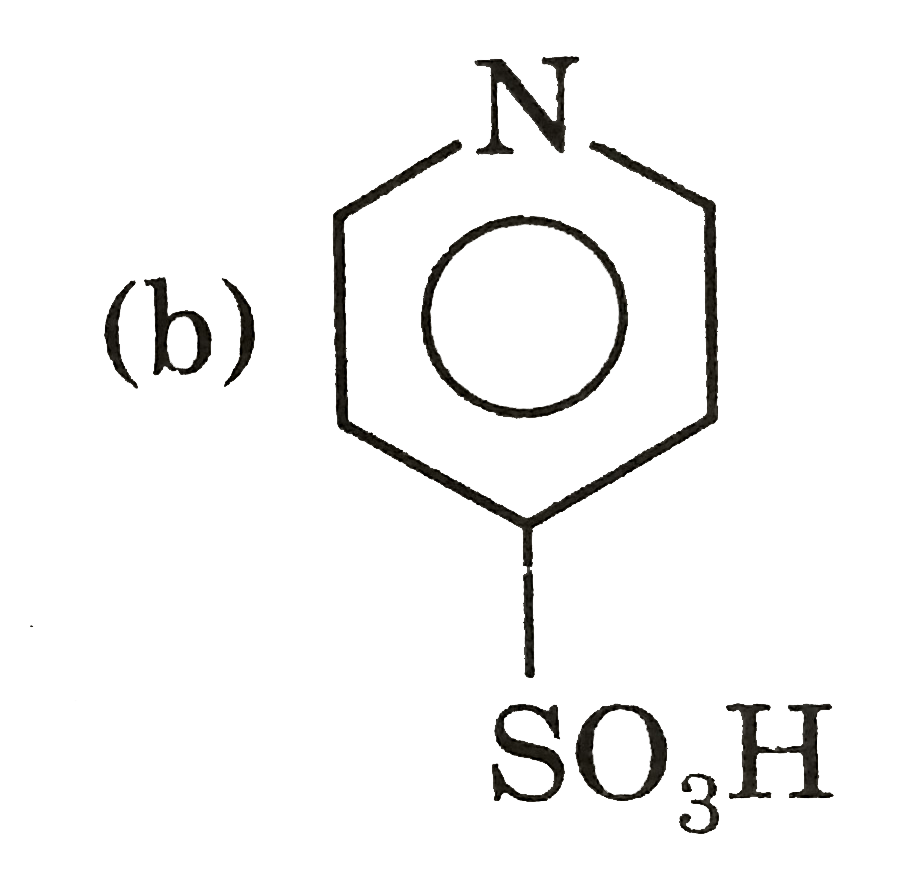 CCH3CH2−NO2DNH2−NH−O∣∣C−NH2
CCH3CH2−NO2DNH2−NH−O∣∣C−NH2Which of the following compounds produces a violet colour on addition of a few drops of FeCl3 solution?
ASalicylic acidBBenzoic acidCtartaric acidDCitric acid
In each of the following pairs, which is more covalent and why?
LiCl and Lil
View SolutionWhich of the following will not give positive test with neutral FeCl3 ?
View SolutionWhich one of the following compounds forms a red coloured solution on treatement with neutral FeCl3 solution ?
View SolutionWhich one of the following compounds forms a red coloured solution on treatement with neutral FeCl3 solution ?
View SolutionWhich of the following can give purple colour with neutral FeCl3
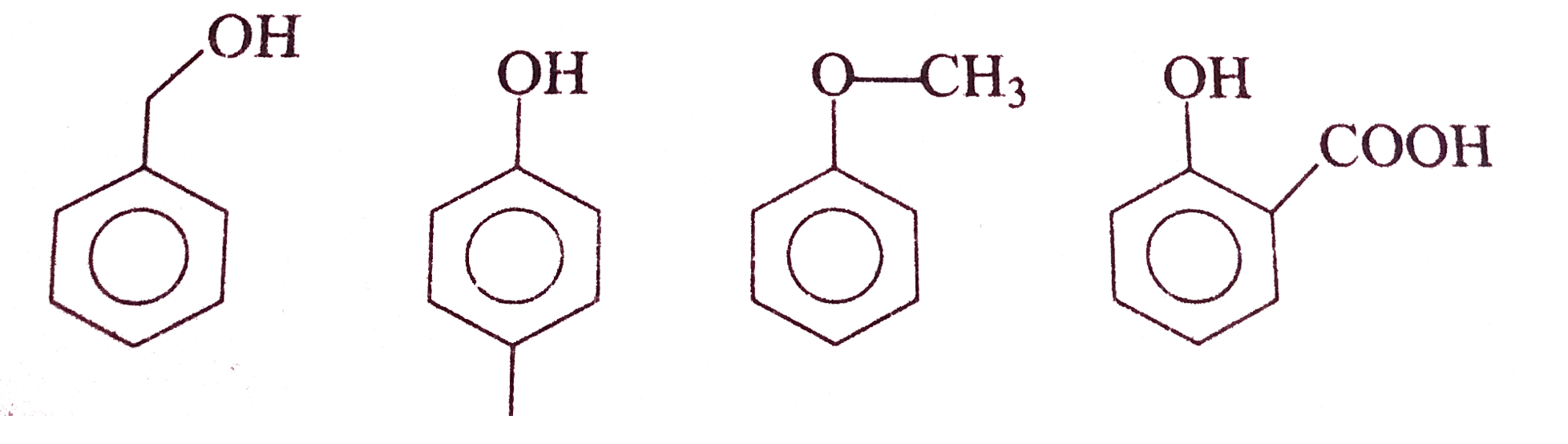
View Solution

