Explain the pressure - volume isotherms of Carbon dioxide Andrew's isotherm.
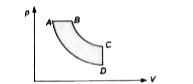
Andrew's isotherms of carbon dioxide at different temperatures is shown in figure From the plots we can infer the following.
(ii) At low temperature isotherms, for example, at 13∘C as the pressure increases, the volume decreases along AB and is a gas until the point B is reached.
(iii) At B, a liquid separates along the line BC, both the liquid and gas co-exist and the pressure remains constant.
(iv) At C, the gas is completely converted into liquid. If the pressure is higher than at C, only the liquid is compressed so, there is no significant change in the volume.
(v) The volume range in which the liquid and gas coexist becomes shorter.
(vi) At the temperature of 31.1∘C the length of the shorter portion is reduced to zero at point P.
(vii) The CO2 gas is liquefied completely at this point. This temperature is known as the liquefaction temperature or critical temperature of CO2.
(viii) At this point the pressure is 73 atm.
(ix) Above this temperature CO2 remains as a gas at all pressure values.

Similar Questions
An ideal gas occupying volume of 2dm3 and a pressure of 5 bar undergoes isothermal and irreversible expansion against external pressure of 1 bat. The final volume of the system and the work involved in the process is
View SolutionPressure - Volume work involved in an isothermal compression is
View SolutionWrite the isothermal relation between pressure and volume of an ideal gas.
View SolutionIn a isothermal process on an ideal gas, the pressure increases by 0.5% . The volume decreases by about.
View SolutionAn ideal gas at 300K is expanded isothermally to double its volume, then pressure of the gas will be
View Solution(A): The internal energy of an ideal gas does not change during an isothermal process
(R ): In isothermal process product of pressure and volume is constant.
View Solutionनीचे दिए गए दाब-आयतन ग्राफ में समआयतनिक, समतापीय एवं समदाबीय भाग क्रमश: है
View Solution

