Which element is more metallic Mg or Al and why?
The correct Answer is:Mg is more metallic due to lower ionisation energy.
Similar Questions
a. Among Cu⊕,Cu2+ and Cu which is the largest in size and why?
b. Which element in periodic table has the highest IE (ionisation energy)?
c. Which element, Mg or Al, is more metallic and why?
View Solutiona. Among Cu⊕,Cu2+ and Cu which is the largest in size and why?
b. Which element in periodic table has the highest IE (ionisation energy)?
c. Which element, Mg or Al, is more metallic and why?
View Solution
Knowledge Check
Which one of the following is most metallic? Na Mg Al Si
ANaBMgCAlDSi
The elements of the third period of the Periodic Table are given below:
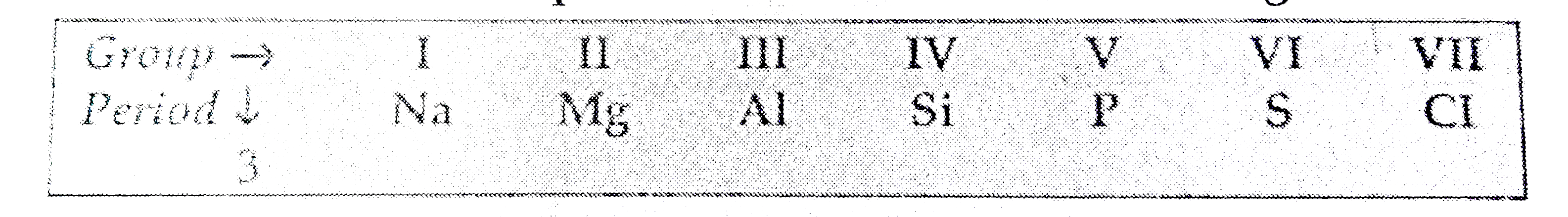
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period 3.
(c) Which is more non-metallic, S or Cl?
(d) Which has higher atomic mass, Al or Cl?
View SolutionThe elements of the third period of the Periodic Table are given below:

(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period 3.
(c ) Which is more non-metallic, S or Cl?
(d) Which has higher atomic mass, Al or Cl?
View SolutionWhich one of Mg and Ca is more metallic?
View SolutionOut of the following elements which is the most non-metallic ?
Na, Mg and Al.
View SolutionOut of the following elements which is the most non-metallic ?
Na, Mg and Al.
View SolutionArrange the following elements in decreasing order of metallic character:
K,Mg,B,Al.
View SolutionArrange the following elements in decreasing order of metallic character:
K,Mg,B,Al.
View Solution

