Which one is good reducing agent (C or CO) for Fe2O3, below 1073 K ?
(ii) Below 1073 k, CO is more effective because the ΔG∘ value for the conversion of CO into CO2 is more negative.
Similar Questions
Knowledge Check
Which is a good reducing agent '?
AYb2+BCe4+CTb4+DLa3+Assertion. Both C and CO can reduce FeO to Fe but at temperatures above 1073 K it is C which is a better reducing agent than CO while below 1073 K, it is CO which is a better reducing agent than C.
Reason. Above 1073 K, ΔfG∘ for CO from C is more negative than that of FeO from Fe while below 1073 K, ΔfG∘CO2 from CO is more negative that that of FeO from Fe.AIf both assertion and reason are true, and reason is not the true explanation of the assertion.BIf both assertion and reason are true, but reason is not the true explanation of the assertion.CIf assertion is true, but reason is falseDIf both assertion and reason are falseCorrect statements form the graph
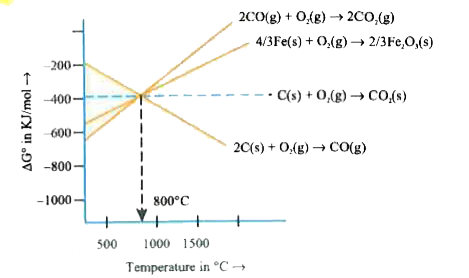
I) Above 1073K, ΔG0 for the formation of Fe2O3 is less negative than ΔG0 for the formation of CO from carbon
II) Above 1073K, Carbon can reduce Fe2O3
III) Below 1073K, CO can reduce Fe2O3
IV) In blast furnace, reduction of Fe2O3 occurs in different temperature ranges with below 1073K by CO (or) above 1073K by carbonAOnly IBI, II onlyCI, II, III onlyDAll
Write the name of reducing agent to obtain iron from Fe2O3 at high temperature.
View SolutionIn the following reaction explain which one is oxidising agent and which one is reducing agent ?
H2O2+O3→2O2+H2O
View SolutionIdentify the reducing agent in the following reactions
Fe2O3+3CO→2Fe+3CO2
View SolutionName the substance oxidised and reduced, and also identify the oxidising agents and reducing agents in the following reactions :
Fe2O3+3CO→2Fe+3CO2
View SolutionCorrect statements from the graph
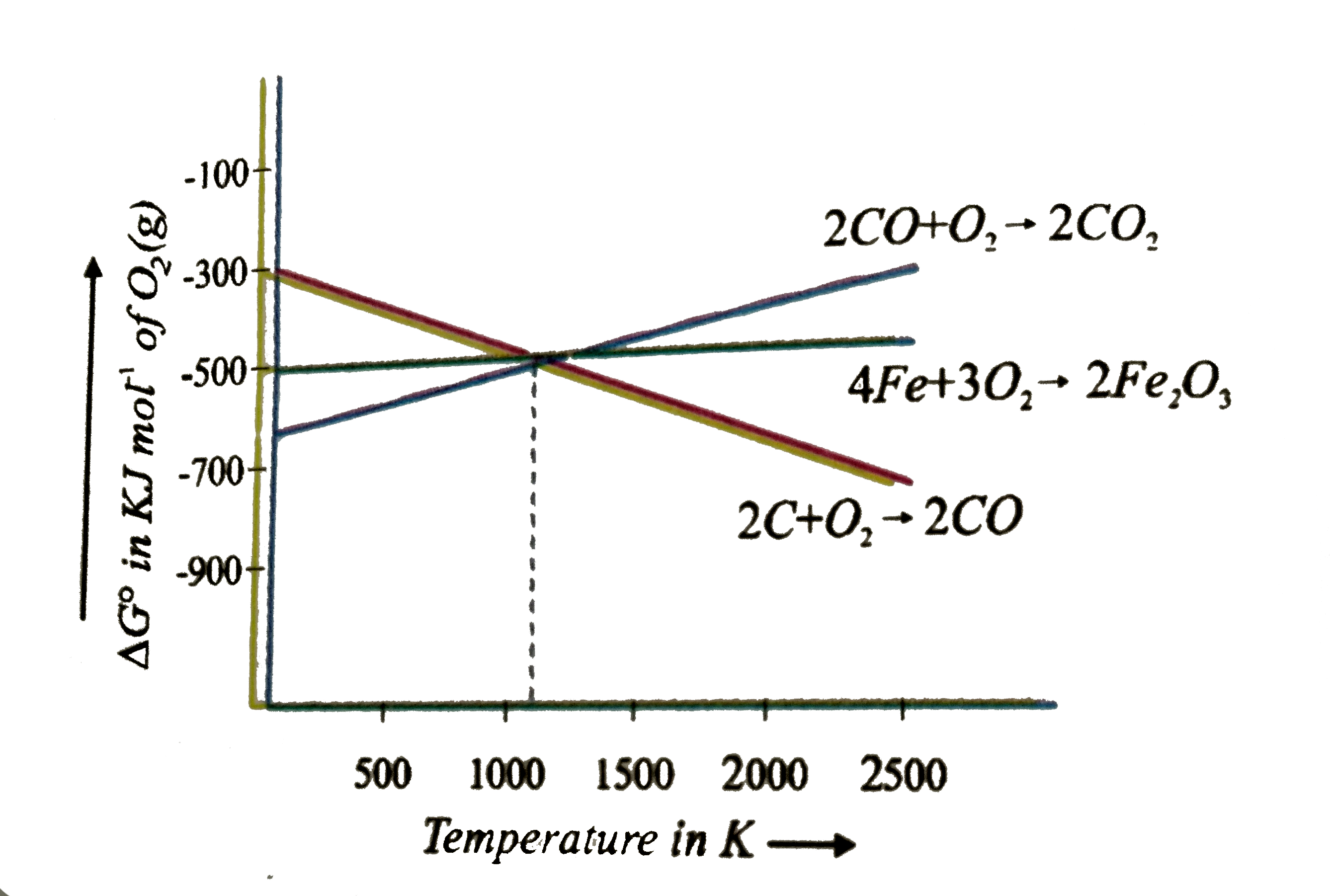
I) Above 1073 K,ΔG0 for the formation of F2O3 is less negative than ΔG0 for the formation of CO from carbon
II) Above 1073K, Carbon can reduce Fe2O3
III) Below 1073K, CO can reduce Fe2O3
IV) In blast furnance, reduction of Fe2CO3 occurs in different temperature ranges with below 1073K by CO (or) above 1073 K by carbon
View Solution

