When temperature is increased, will ionic product of water increase or decrease? Give reason to justify your answer.
Text Solution
With increases in temperature the ionic product of water will increases since the concentration of H3O+andOH− ions will increase.
|
Similar Questions
Knowledge Check
Ionic product of water increases, if
AH+ ions are addedBOH ions are addedCTemperature decreasesDTemperature increasesIonic product of water increases, if
APressure is reducedBTemperature increasesCOH− is addedDTemperature increasesWith decrease of temperature, ionic product of water
AincreasesBdecreasesCremains the sameDmay increase or decrease
Will ionic product of water increase or decrease if temperature is increased ?
View SolutionConsider the following circuit :
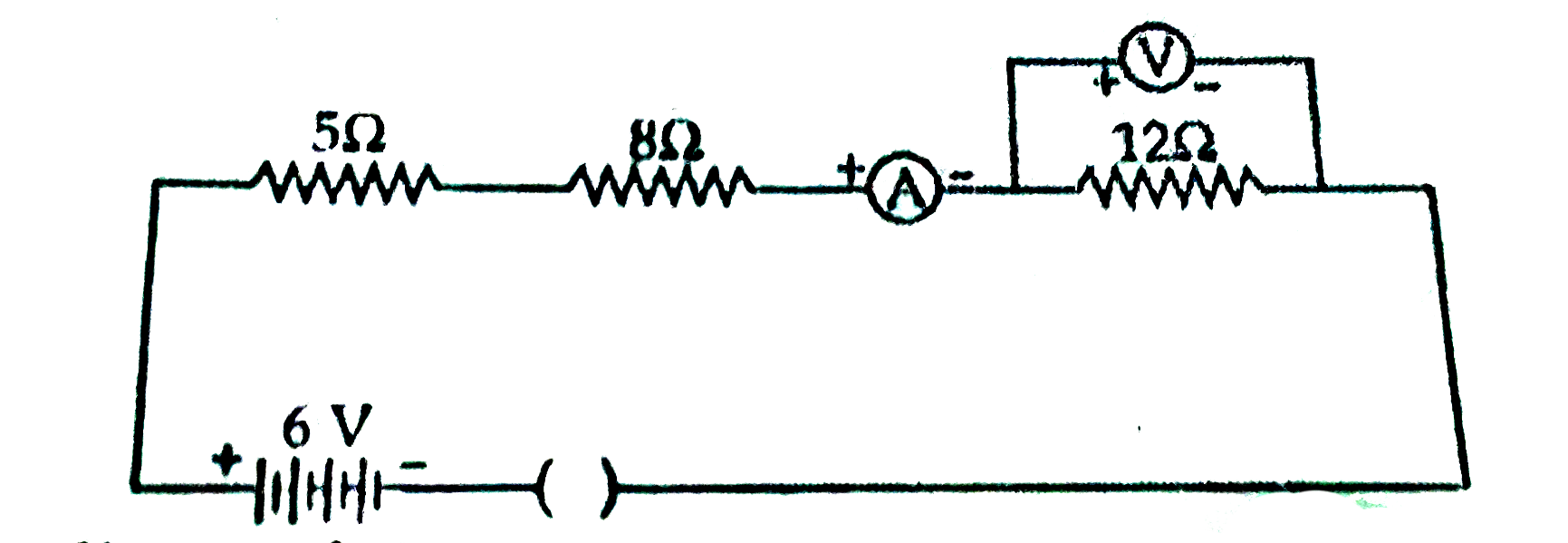
What would be the readings of the ammeter and the voltmeter when key is closed ? Give reason to justify your answers .
View SolutionIncrease in temperature decreases the conductivity of metallic conductors. Give a reason.
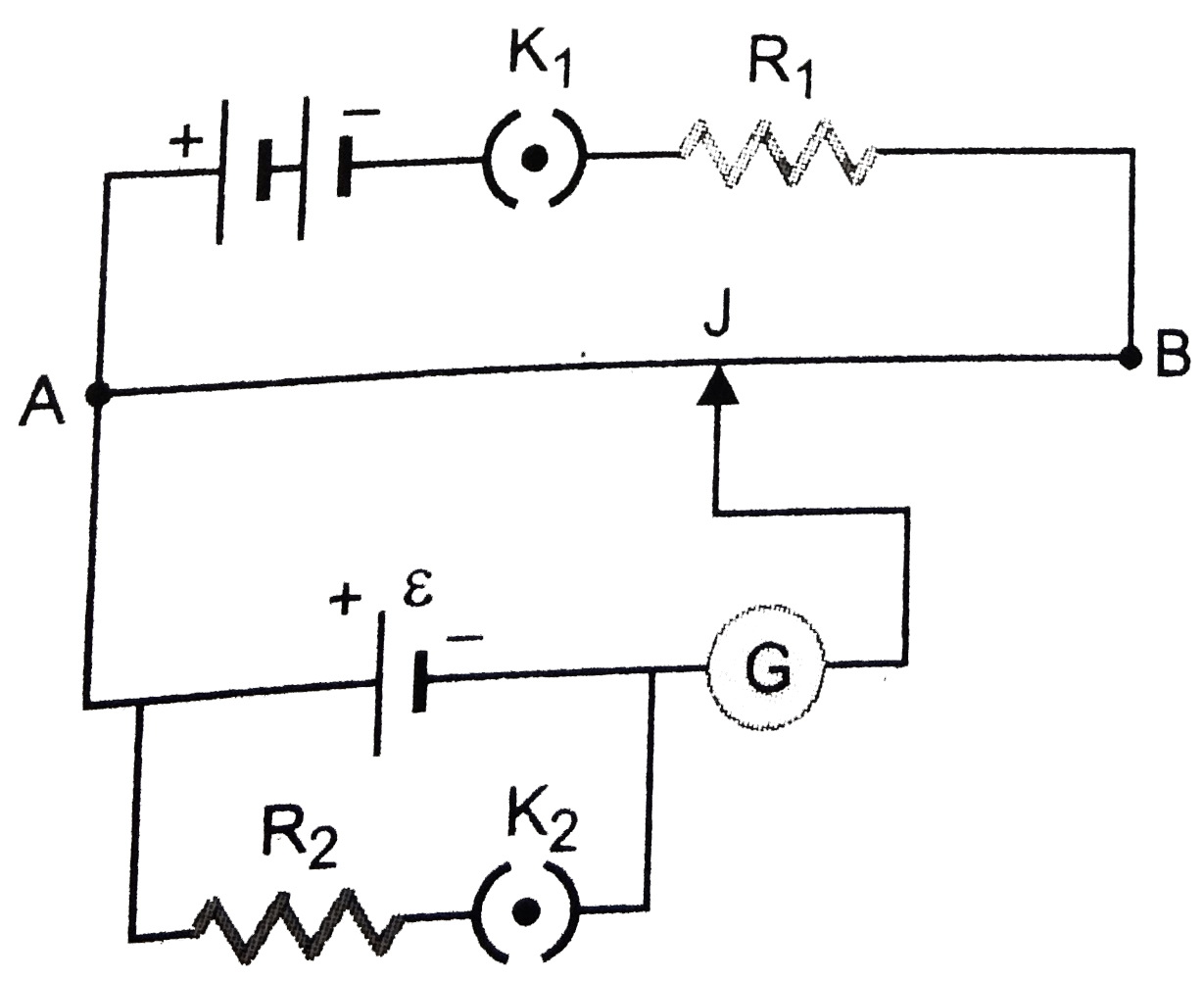
View SolutionFor the circuit would the balancing length increase, decrease or remain the same if (i) R1 is decreased (ii) R2 is increased, without any change (in each case) in the rest of the circuit ? Justify your answer in each case.
View SolutionAssertion: The value of ionic product of water (Kw) increases on increasing the temperature.
Reason: The ionisation of water increases with an increase in temperature.
View Solution

