The compound with α - hydrogen is _______
A
HCHO
B
C6H5CHO
C
C6H5COC6H5
D
CH3COCH3
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
The α-hydrogen atom attached to the carbonyl group is acidic in nature because the carbanion which is left gets resonance stabilished. As a result, these compounds take part in the aldol condensation reactions. The carbanion acts as a nucleophile in these reactions. The compounds in which α-hydrogen is not acidic take part in the Cannizzaro's reaction. In this reaction, one molecule of the carbonyl compound under consideration is reduced to primary alcohol while the other is oxidised simutaneously to the carboxylic acid.
An organic compound 'A' of the molecular formula C5H10Cl2 is hydrolysed to compound 'B' C5H10O which gives an oxime with hydroylamine and yellow precipitate with a mixture of iodine and sodium hydroxide. The compound 'A' should be :ACH3CH2CCl2CH2CH3BCH3CH2CH2CCl2CH3CCH3CH2CH2CH2CHCl2DCH3CH2CH2CHClCH2Cl.In the presence of small amount of phosphorous, aliphatic carboxylic acids reacts with chlorine or bromine to yeild a compound in which α-hydrogen has been replaced by halogen. This reaction is known as
AWolf-Kishner reactionBEtard reactionCRosenmund reactionDHell-Volhard-Zelinsky reactionIn the presence of a small amount of phosphorous, aliphatic carboxylic acids react with chlorine or bromine to yield a compound in which α- hydrogen has been replaced by halogen. This reaction is known as :
AEtard reactionBHell-Volhard-Zelinsky reactionCWolff-Kischner reactionDRosenmund reaction
Match the compounds with type of hydrogen bond.

View Solution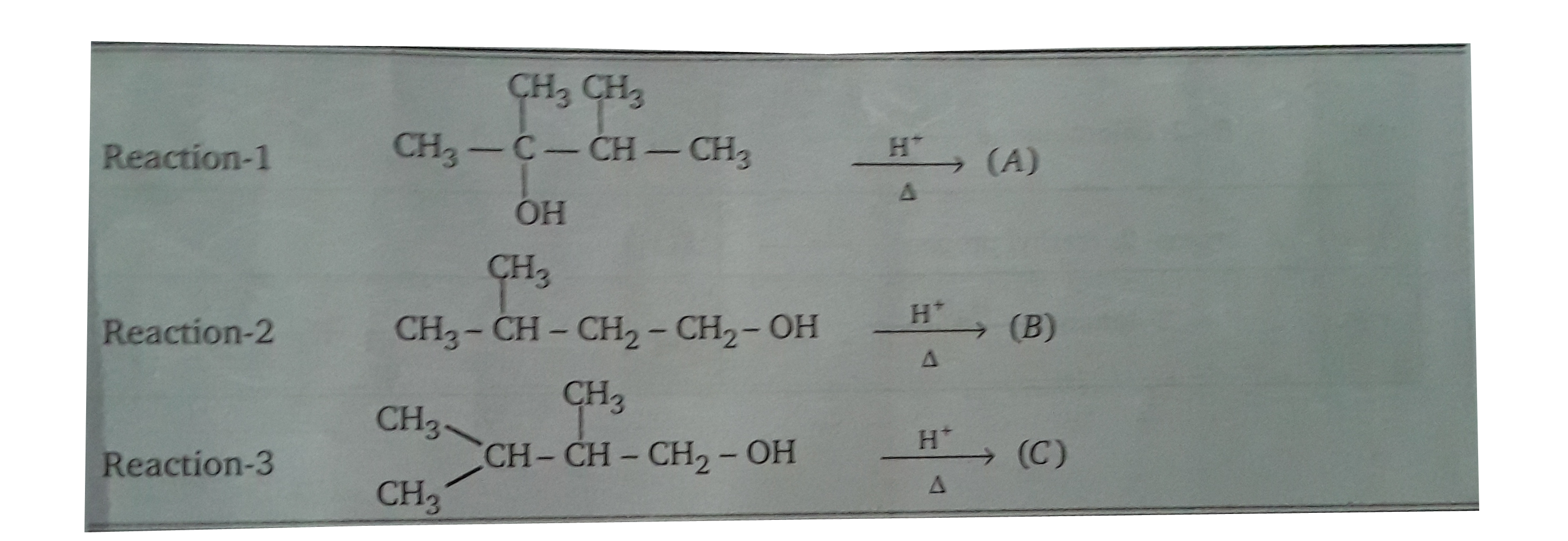
sum of α -hydrogen is (A+B+C=)
View SolutionThe compound containing hydrogen bond is-
View SolutionWhich compound has hydrogen bonding
View SolutionA compound possessing α -hydrogen atom, in the presence of dilute alkali forms β -hydroxy aldehyde. This product on heating with dilute acid forms an unsaturated crotonaldehyde. The compound is
View Solution

