Explain Stephen's reaction.
Text Solution
When alkylcyanides are reduced using SnCl2/HCl, imines are formed, which on hydrolysis gives corresponding aldehyde.
CH3−C≡NSnCl2/HCl−−−−−−→(H)CH3−CH≡NHH3O+−−−→CH3−CHO
|
Topper's Solved these Questions
- CARBONYL COMPOUNDBOOK - SURA PUBLICATIONCHAPTER - CARBONYL COMPOUNDEXERCISE - LONG ANSWER
27 Videos
- CARBONYL COMPOUNDBOOK - SURA PUBLICATIONCHAPTER - CARBONYL COMPOUNDEXERCISE - PROBLEMS FOR PRACTICE
19 Videos
- CARBONYL COMPOUNDBOOK - SURA PUBLICATIONCHAPTER - CARBONYL COMPOUNDEXERCISE - VERY SHORT ANSWER
62 Videos
- BIOMOLECULESBOOK - SURA PUBLICATIONCHAPTER - BIOMOLECULESEXERCISE - UNIT TEST
9 Videos
- CHEMICAL KINETICS BOOK - SURA PUBLICATIONCHAPTER - CHEMICAL KINETICS EXERCISE - UNIT TEST (ANSWER THE FOLLOWING IN DETAIL)
2 Videos
Similar Questions
Knowledge Check
Stephen's reduction convert
Aacyl chloride to aldehydeBcyanide to aldehydeCcyanide to carboxylic acidDcyanide to ketonesSelect the reagent used and the intermediate formed in Stephen reaction.
A B
B C
C D
D
What is the reagent used in Stephen reaction ?
AChromyl chlorideBEthanoyl chlorideCSnCl2 and HClDCadmium chloride
Stephen Reduction.
View SolutionWhat is Stephen reaction ?
View SolutionComplete the following Mendius and Stephen's reductions:
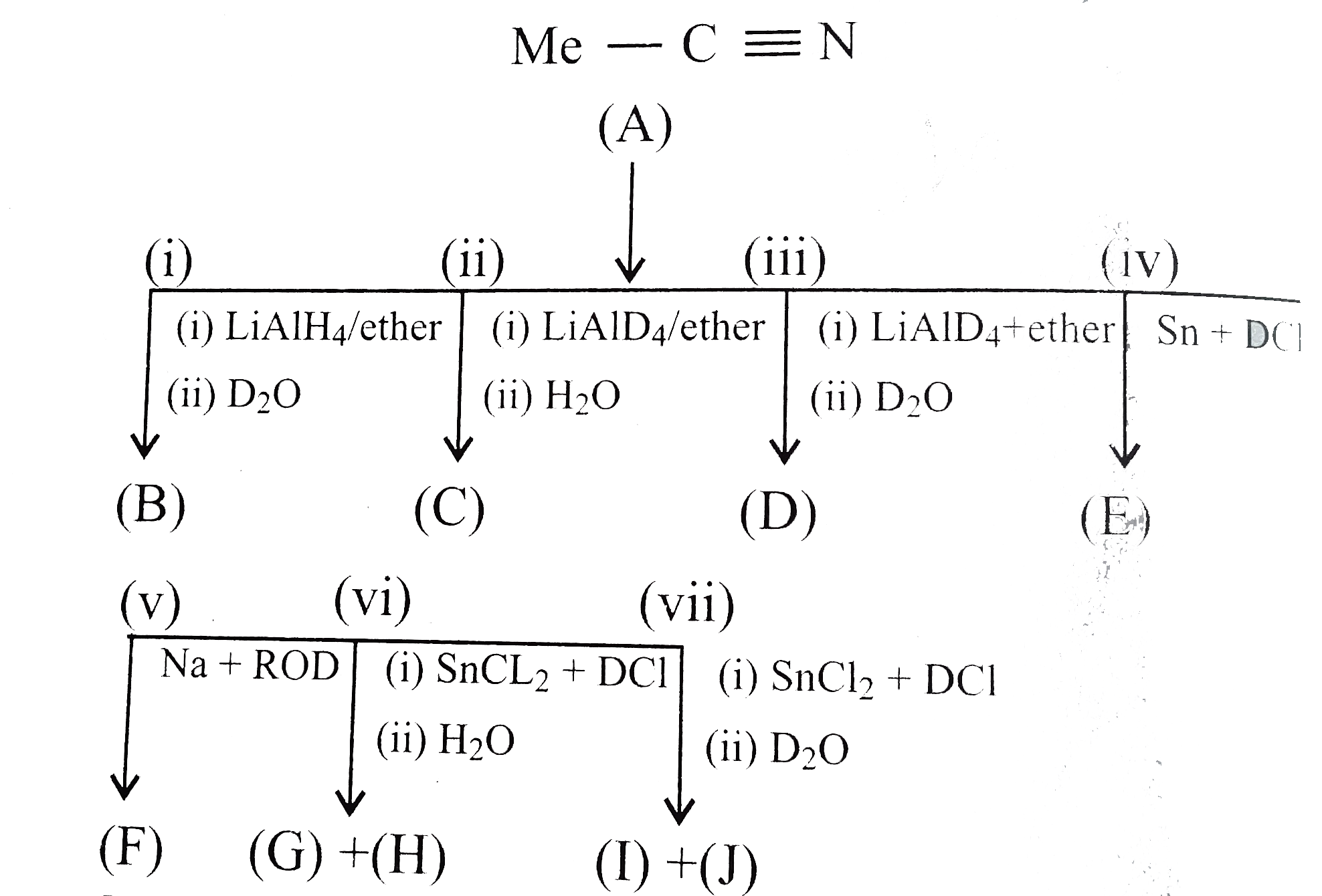
View SolutionWrite the equation involved in the following reactions:
(i) Stephen reaction
(ii) Etard reagent
View SolutionWhich of the following is an example of Stephen reaction?
View Solution

