Explain the mechanism of Cannizaro reaction.
Text Solution
Cannizaro reaction involves three steps.
Step 1 : Attack of OH - on the carbonyl carbons.

Step 2 : Hydride ion transfer

Step 3 : Acid - base reaction.
C6H5−O∣∣C−OH+C6H5CH2O−+Na+Proton−−−−→exchangeC6H5−O∣∣C−ONaSodium benzoate+C6H5CH2OHBenzyl alcohol
Cannizaro reaction is a characteristic of aldehyde having no α-hydrogen.
|
Similar Questions
Knowledge Check
Which will give Cannizaro reaction ?
ACl3C−CHOBC6H5CHOC(CH3)3−C−CHODAll of the aboveMechanism of cannizzaros reaction of benzaldehyde is
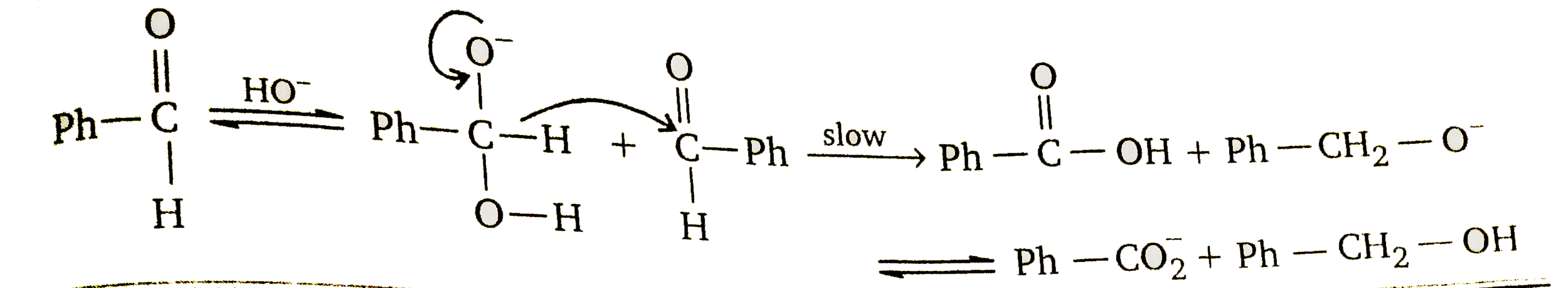
Q. Cannizaro's reaction isAReductionBDisproportionation reactionCOxidationDIon-exhange reaction.Mechanism of cannizzaros reaction of benzaldehyde is
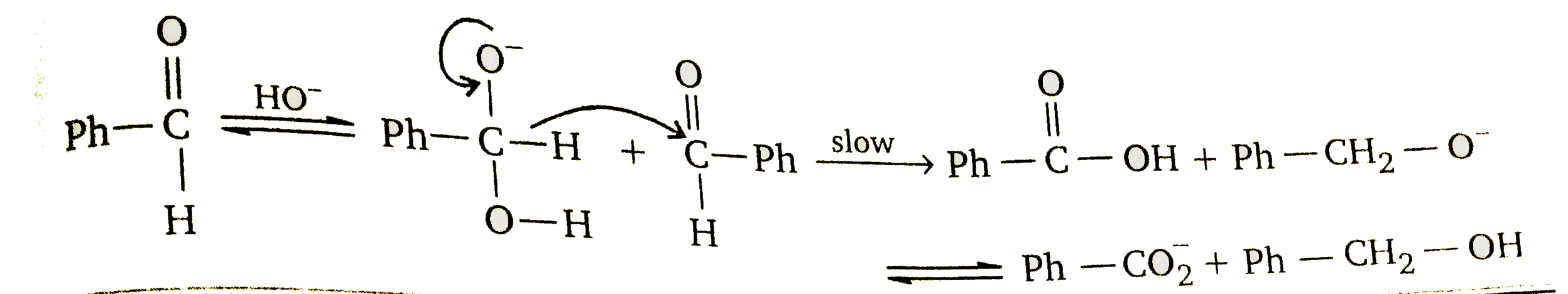
Q. Which of the following is best hydride donor in cannizaro's reaction?A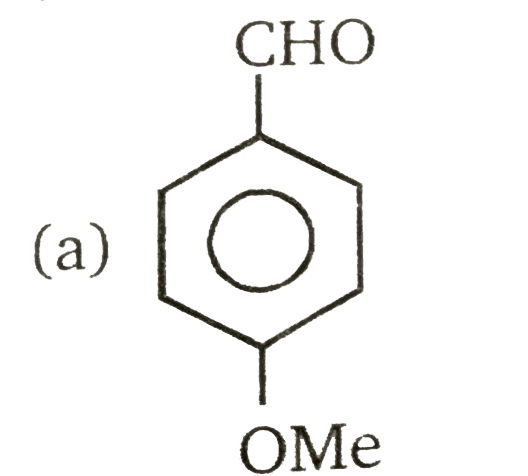 B
B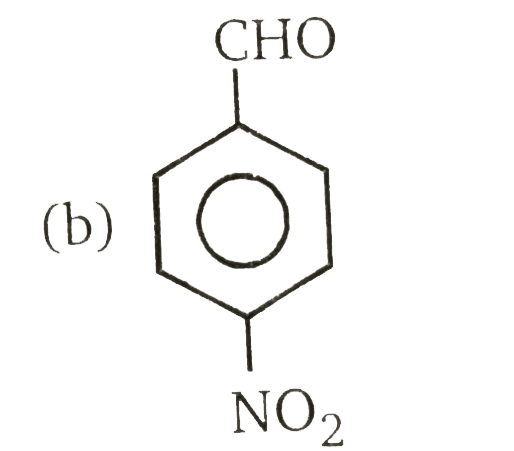 C
C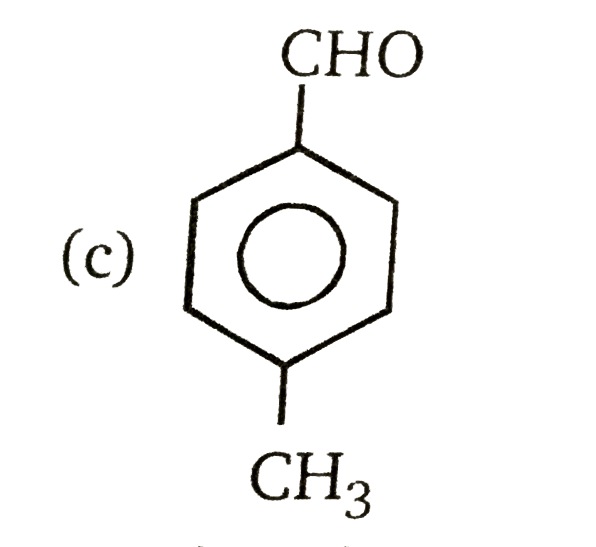 D
D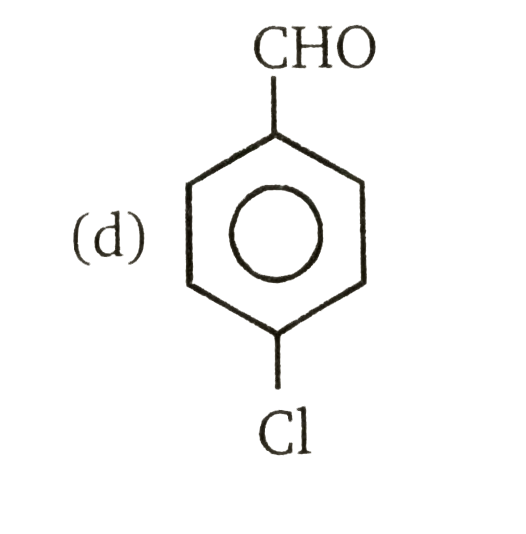
Explain the mechanism of the following reaction:
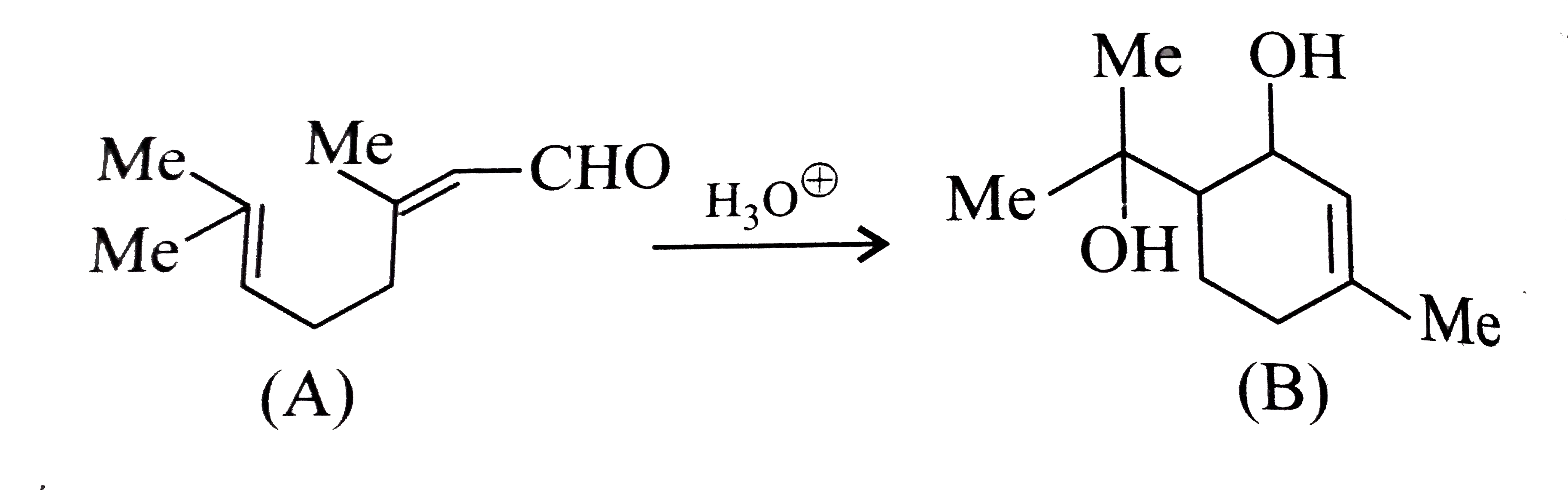
View SolutionGive four important reactions of benzene and explain the mechanism of any one of these reactions.
View SolutionExplain the reaction mechanism of addition of HCN to benzaldehyde ?
View SolutionExplain the mechanism of electrophilic addition reactions of alkenes.
View SolutionMechanism of cannizzaros reaction of benzaldehyde is
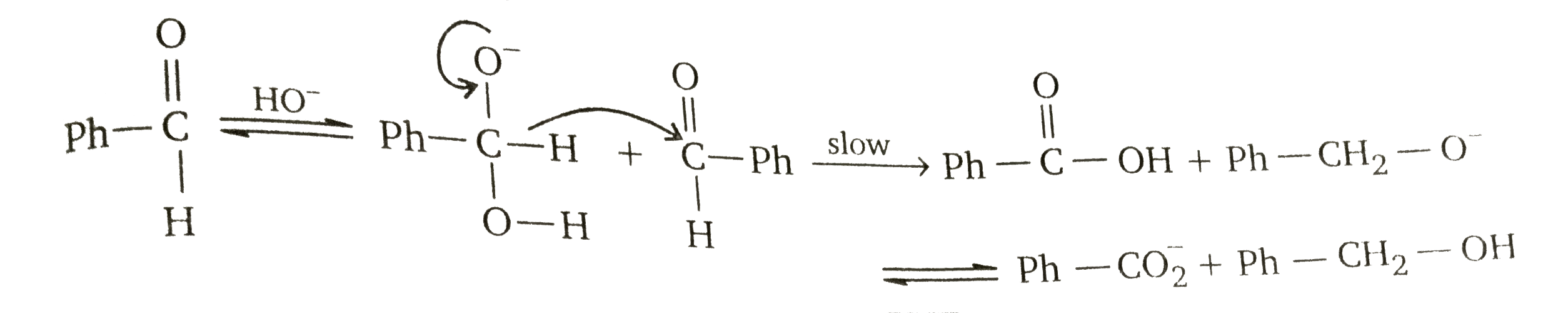
Q. Which of the following reactants can undergo cannizaro's reaction?
View Solution

