The measurement of the amount of heat energy released or abosrbed by a thermodynamic system during the heating process is called ______. Solution in Tamil
Heat capacity
Enthalpy
Entropy
Calorimetry
The correct Answer is:D
Step by step video, text & image solution for The measurement of the amount of heat energy released or abosrbed by a thermodynamic system during the heating process is called ______. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Thermodynamics deals with the concept of heat and the exchange of heat energy.
Obtain the expression for the work done during an adiabatic process.
View SolutionThe amount fo heat given to a system in a cyclic thermodynamic process
View SolutionThe instrument by which heat absorbed or heat released is measured is called
View SolutionCalculate the heat abosrbed by a system in going through the cyclic process show in Fig.
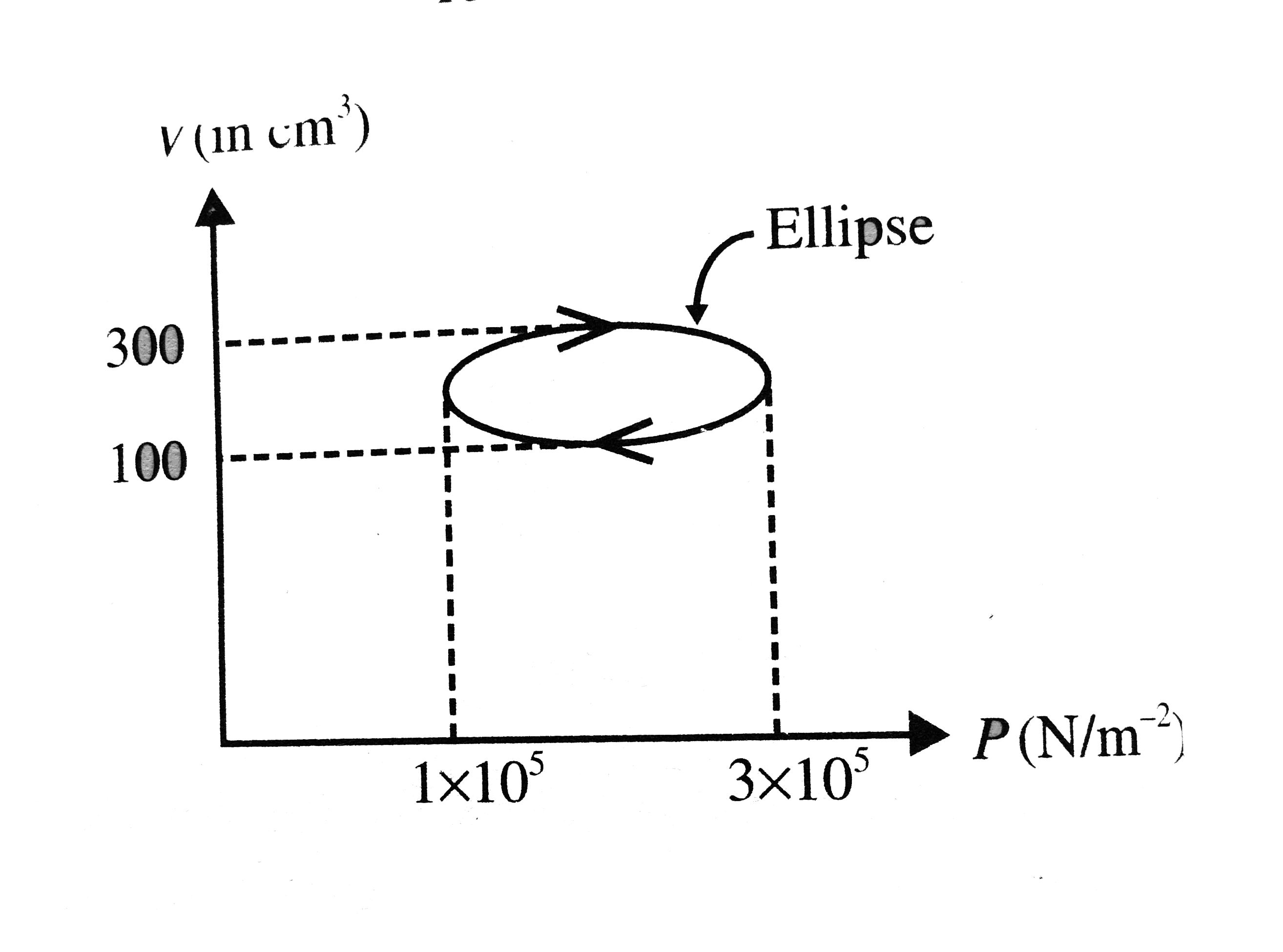
View SolutionIn a thermodynamic cyclic process ABC, internal energy at A is 10J and at B is 70J. Heat given in process B to C is 100J, then heat released or absorbed in process C to A is
View SolutionThe amount of heat given to a system in a cyclic thermodynamical process-
View SolutionWhat is the internal energy of the system, when the amount of heat Q is added to the system and the system does not do any work during the process?
View SolutionIn a thermodynamic system,internal energy decreases by 300J when it is doing 350J of work.What net heat is taken by system in the process?
View Solution
