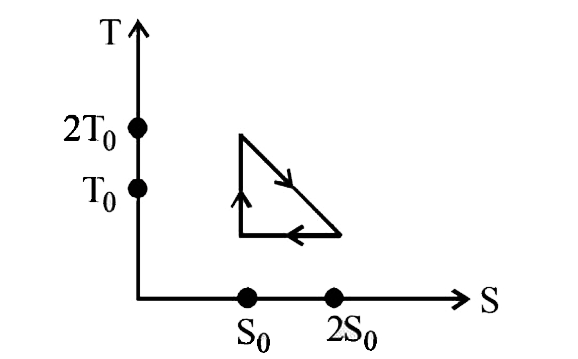
The temperature - entropy diagram of a reversible cycle is given in figure. Calculate its efficient .

Text Solution
Heat produced by the system
Q1=T0S0+12T0S0=32T0S0
Q2=T0(2S0−S0)=T0S0
Q3=0
∴ Efficiency η=1−Q2Q1
=1−T0S032T0S0
=1−2T0S03T0S0⇒1−23=3−23=13
∴ Efficiency η=13
|
Similar Questions
Knowledge Check
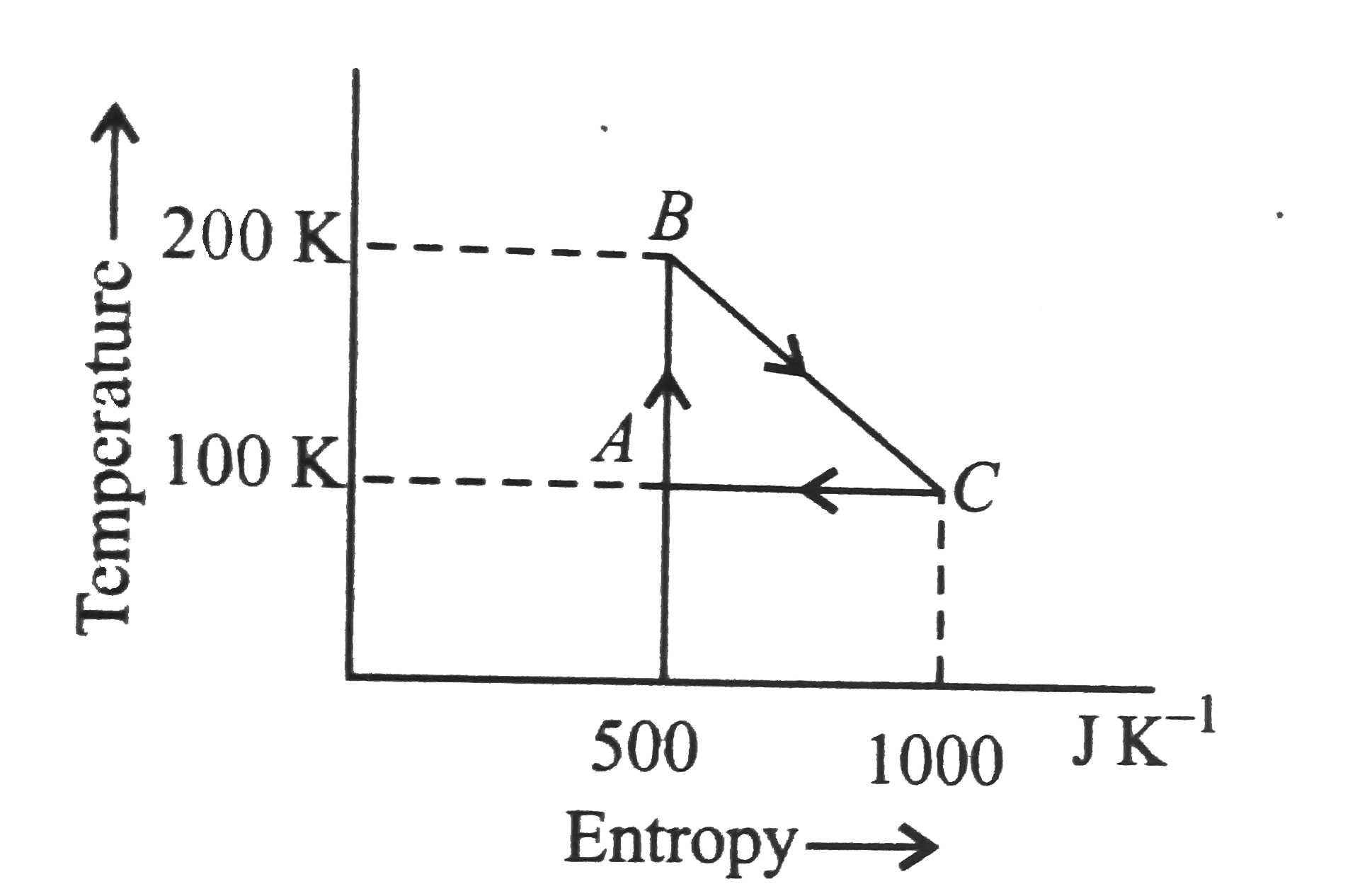
The temperature -entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
 A14B12C23D13
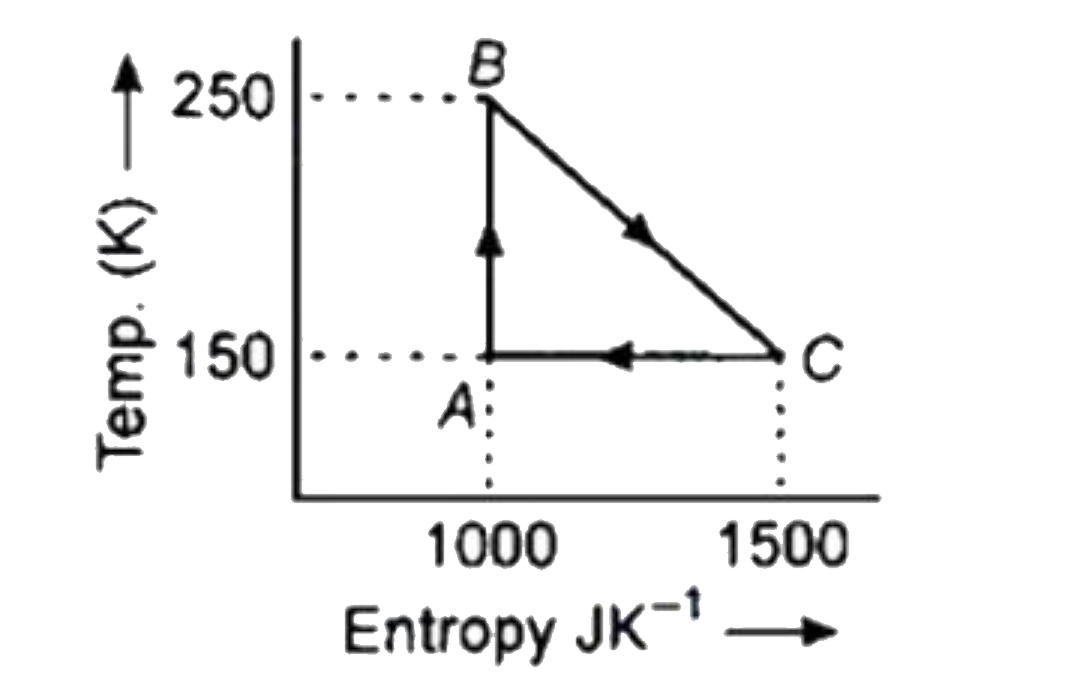
A14B12C23D13The efficiency of the reversible cycle shown in the given figure is
 A33.33%B56%C66%D16.7%
A33.33%B56%C66%D16.7%The efficiency of the reversible cycle shown in the figure will be
 A33.33%B56%C66%D25%
A33.33%B56%C66%D25%
The efficiency of the reversible cycle shown in the figure will be

View SolutionPlot the Carnot cycle in the T-S coordinates and calculate its efficiency.
View SolutionOne mole of ideal monatomic gas is carried through the reversible cyclic process as shown in figure. Calculate the max temperature attained by the gas during the cycle.
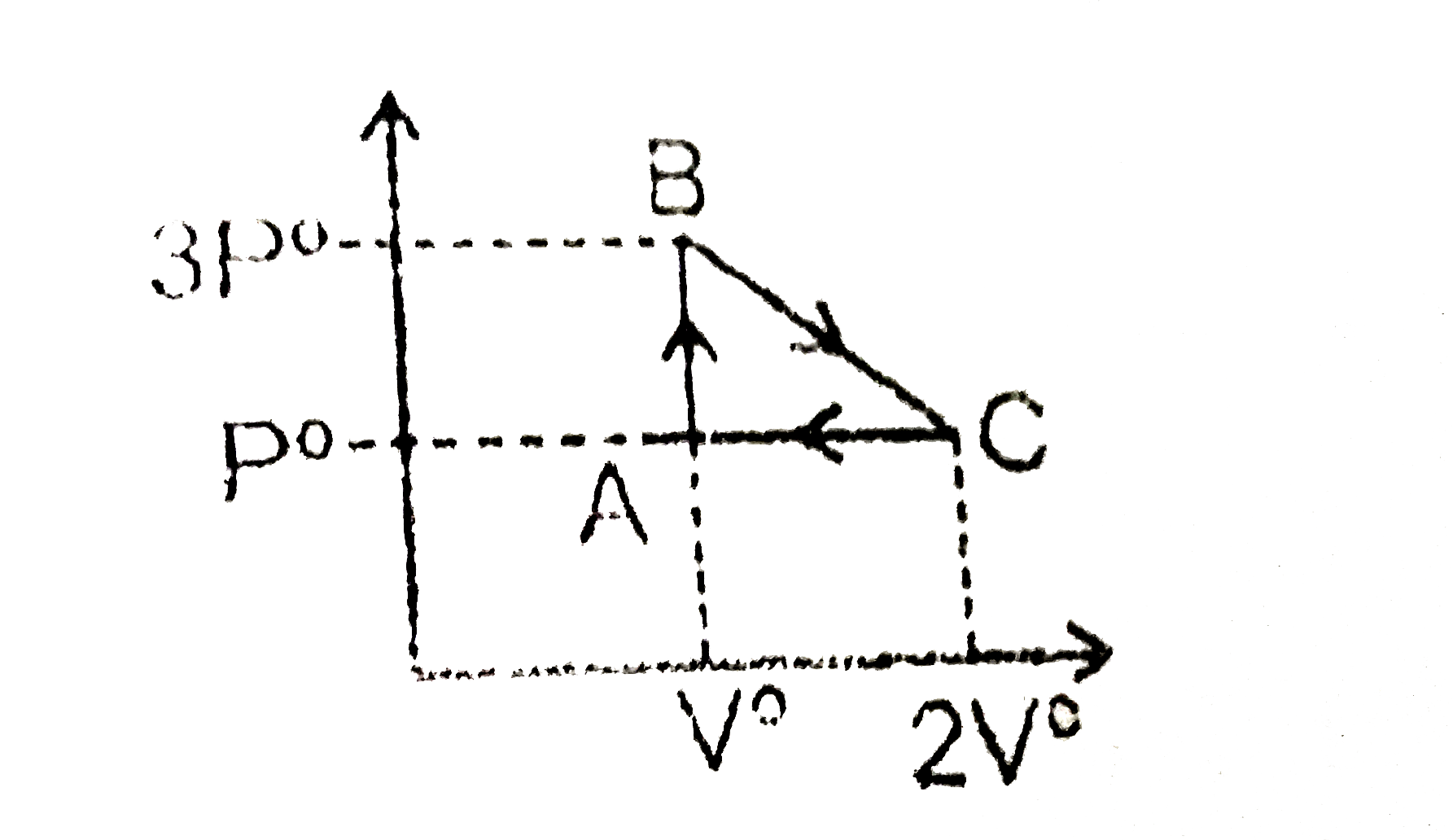
View SolutionOne mole of ideal monatomic gas is carried through the reversible cyclic process as shown in figure. Calculate the max temperature attained by the gas during the cycle.

View SolutionAn ideal gas is taken reversibly around the cycle a-b-c-d-a as shown on the T (temperature) – S (entrophy) diagram

The most appropriate representation of above cycle on a U (internal energy) – V (volume) diagrame is
View Solution

