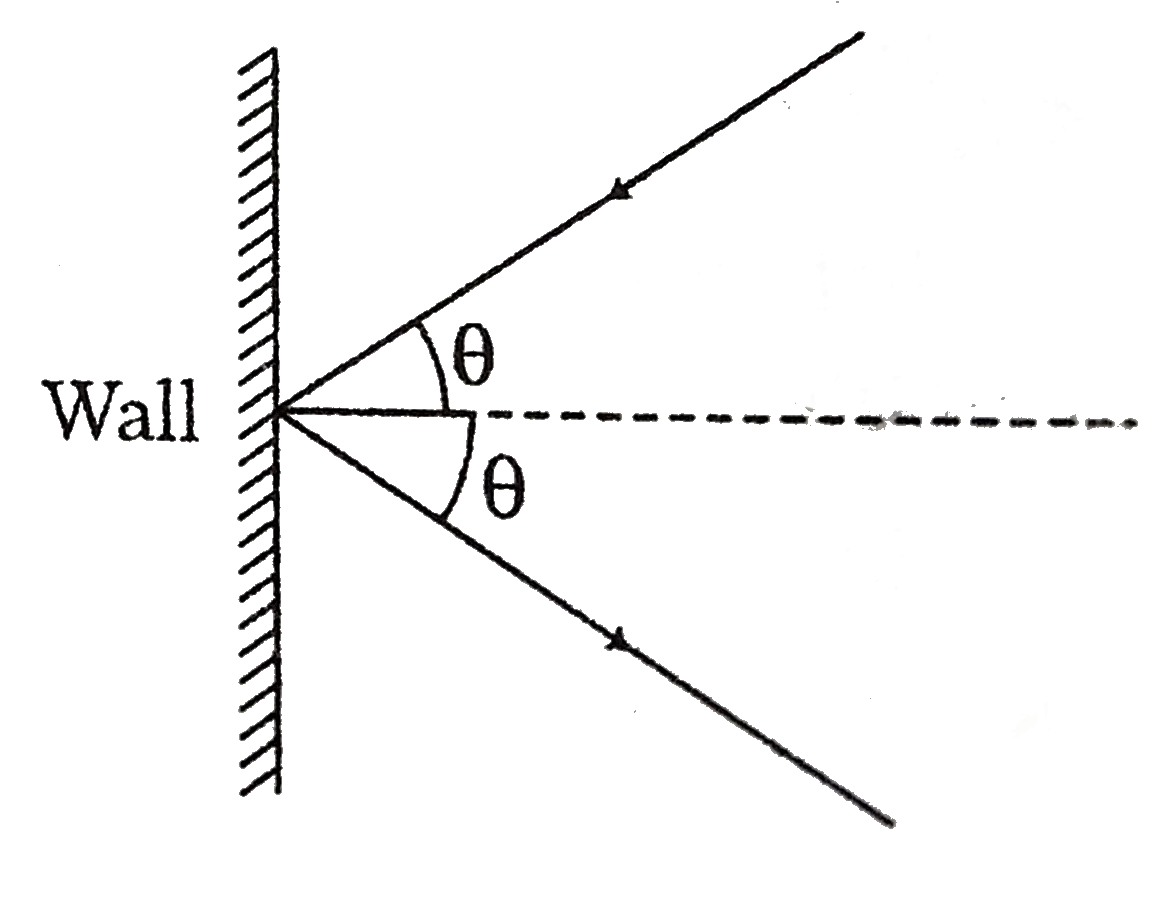
If 1020 oxygen molecules per second strike 4cm2 of wall at an angle of 30∘ with the normal when moving at a speed of 2×103ms−1, find the pressure exerted on the wall. (mass of 1O2 atom = 2.67×10−26kg) Solution in Tamil
Mass of 1O2 atom =2.67×10−26kg
Mass of 1020O2 atom = 2.67×10−26kg
=26.72×10−27×1020
Momentum P = mv
=26.72×10−7×8×2×103
=427.5×10−4kgms−1
Component of momentum normal to wall is 30∘
=427.5×10−4×cos30∘
=427.5×10−4×√32
=370.2×10−4kgms−1
Pressure = FA=Force (change in momentum)area
=370.2×10−44×10−4
P=92.55Nm−2
Similar Questions
The mass of a hydrogen molecule is 3.32xx10^(-27) kg. If 10^(23) hydrogen molecules strike, per second, a fixed wall of area 2 cm^(2) at an angle of 45^(@) to the normal, and rebound elastically with a speed of 10^(3) m/s, then the pressure on the wall is nearly
View SolutionWhen a ball of 0.5 kg mass moving with a speed of 20 ms−1 rebounds after striking normally a ferfectly elastic wall. Find the change in momentum.
View SolutionIf 1020 oxygen molecules per second strike 4cm2 of wall at an angle of 30∘ with the normal when moving at a speed of 2×103ms−1, find the pressure exerted on the wall. (mass of 1O2 atom = 2.67×10−26kg)
View SolutionThe mass of H2 molecule is 3.32×10−24 g .If 1023 hydrogen molecules per second strike 2 cm2 of wall at an angle of 45∘ with the normal,when moving with a speed of 105 cm/s,the pressure exerted on the wall is nearly
View SolutionA ball of mass 0.4 kg moving with a uniform speed of 2 ms. strikes normally a wall and rebounds. Assuming the collision to be elastic and the time of contact of the ball with the walls is 0.4 s, find the magnitude of force exerted on the ball.
View SolutionThe mass of H_2 molecule is 3.32 xx 10^(-24) g. If 10^(23) hydrogen molecules per second strike 2 cm^(2) of wall at an angle of 45° with the normal, when moving with a speed of 10^(5) cm/s, the pressure exerted on the wall is nearly.
View SolutionPassage-II : The mass of hydrogen molecule is 3.32 xx 10^(-27) kg. If 10^(23) hydrogen molecules strike a fixed wall of area 2cm^(2) at an angle 45^(@) to the normal, per second and rebound elastically with a speed of 10^(3) m//s then The pressure exerted on the wall by the hydrogen gas is
View SolutionThe mass of hydrogen molecule is 3.32×10−27 kg. If 1023 hydrogen molecules strick per second at 2 cm2 area of a rigid wall at an angle of 45∘ from the normal and rebound back with a speed of 1000 ms−1, then the pressure exerted on the wall is
View Solution
