A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
HgO
Pb3O4
(NH4)2Cr2O7
Hgl2
The correct Answer is:D
The red solide is Hgl2
Hgl2(Red solide)+2Kl→K2[Hgl4](soluble)
Hgl2heat−−→Hg(l)(Droplets)+I2(g)(Violet fumes)
Topper's Solved these Questions
Similar Questions
A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating rod solid in a test tube produces violet coloured fumes and droplets of metal appear on the cooler parts of test tube. The red solid is
View SolutionIn the following four test tubes, some metals are in contact with certain salt solutions. After the experiment, in which of the test tubes does the solution becomes colourless and a powdery red mass is deposited at the bottom of the test tube ?
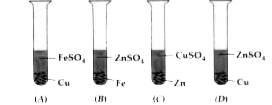
View Solution
Knowledge Check
A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The rod solid is:
AHgI2BHgOCPb3O4D(NH4)2Cr2O7A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The rod solid is:
A(NH4)2Cr2O7BHgI2CHgODPb3O4A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The rod solid is:
A(NH4)2Cr2O7BHgI2CHgODPb3O4
A red solid is insoluble in water.However it becomes soluble if some KI is added to water.Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube.The red solid is :
View SolutionA red coloured solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating red solid in a test tube results in the liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
View SolutionA red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The rod solid is:
View SolutionA red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating rod solid in a test tube produces violet coloured fumes and droplets of metal appear on the cooler parts of test tube. The red solid is
View SolutionA solid is a compound of group I element and it gives a bright red colour in the flame test. The solid is
View Solution

