A hydrocarbon of molecular weight 72gmol−1 has a 2-methyl group. What is the IUPAC name? Also drawn its bond-line structure?
Let the molecular formula of hydroxarbon =CxHy
Molar mass =72g mol−1 (given)
The maximum value of x=5
∴5×12+Y(1)=72
Y=72−60=12
The molecular formula =C5H12
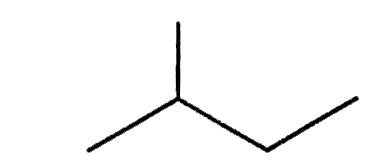
Structural formula :
Ch3 |CH3−CH−CH2−CH3 2-Methylbutane
Bond Line notation
Topper's Solved these Questions
Similar Questions
A hydrocarbon of molecular mass 72 g mol−1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
View SolutionA hydrocarbon of molecular mass 72 g mol−1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
View Solution
Knowledge Check
A compound with molecular formula C6H14 has two tertiary carbons. Its IUPAC name is
An-hexaneB2-methyl pentaneC2,3-dimethyl butaneD2,3-dimethyl pentaneA carbohydrate Xhaving molecular weight 180gmol−1 has one primary alcoholic group and four secondary alcoholic groups. It reacts with acetic anhydride to form pentaacetate. The molecular weight of pentaacetate formed is
A180B210C390D42A compound with molecular formula C6H14 has two tertiary carbons. Its IUPAC name is
An-hexaneB2-methylpentaneC2, 3-dimethylbutaneD2, 3-dimethylpentane
Explain hybridisation in XeF2. Also draw its molecular structure.
View SolutionA hydrocarbon with lowest molecular weight has only onne chiral carbon. Find its molecular weight.
View SolutionA hydrocarbon has the molecular mass 70. Write all the possible structural isomers and give their IUPAC names
View SolutionTyrosine is one of the amino acids present in protein. Its content in the protein is 0.22% and its molecular weight is 181gmol−1. What is the lowest molecular weight of the protein?
View SolutionA compound C6H14O2 has two tertiary groups. The IUPAC name of the compound is
View Solution

