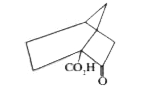
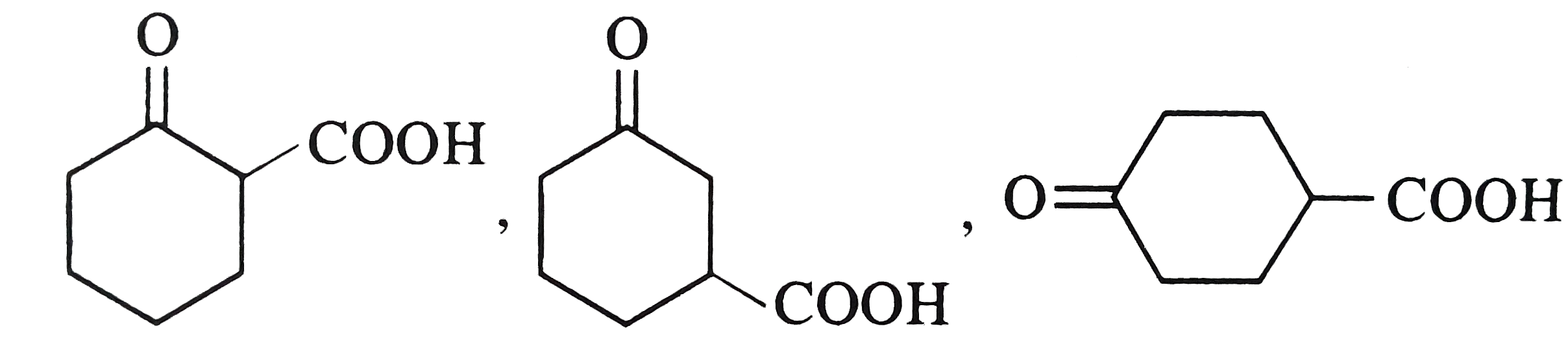
Which of the following β-keto carboxylic acid does not undergo decarboxylation on heating ?
A

B

C

D
None of these
Video Solution
Text Solution
The correct Answer is:A
(a) β-Keto acids undergo decarboxylation through the keto form via a cyclic T.S. which results in the formation of the enol form of the ketone product . The enol then charges to the more stable keto form . If decarboxylation of the given β-Keto acid could follow this route , then :

If we draw the acid as shown in(I) , it can be seen that the methylene group forms a bridge , which is perpendicular to the cyclohexane ring .

The enol product would have a double bond in the 1,2-position . Thus C-1 is sp2 -hybridised and therefore the bond leading to the methylene bridge (1,3) would have to be coplanar with the groups joined to C-2 in the enol , i.e., the bridge would have to flattern. This would require the normal valency angles (~109.5∘) to change to ~120∘ . Even if a compromise situation occured , i.e., all bond angles involved (∠s1,3and4) underwent change , the resulting strain would be very large , too large to be expected for it to occur. Hence , it is reasonable to argue that since decarboxylation does occur , it does so through a route requiring a much higher activation energy than that required for the 'usual' route . Hence the difficulty of decarboxylation . (Bredt's rule )
|
Topper's Solved these Questions
- ALDEHYDES AND KETONES Book:MS CHOUHANChapter:ALDEHYDES AND KETONES Exercise:Level 1 (Q.121 To Q.150)
Explore 1 Video
- ALDEHYDES AND KETONES Book:MS CHOUHANChapter:ALDEHYDES AND KETONES Exercise:Level 2
Explore 7 Videos
- ALCOHOLS FROM CARBONYL COMPOUNDSBook:MS CHOUHANChapter:ALCOHOLS FROM CARBONYL COMPOUNDSExercise:Additional Objective Questions (Multiple Correct Choice Type )
Explore 1 Video
- ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUPBook:MS CHOUHANChapter:ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUPExercise:ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)
Explore 17 Videos
Similar Questions
Knowledge Check
Which of the following carboxylic acids do not undergo decarboxylation simply on heating?
AF3C−O||C−OHBH2C=CH−O||C−OHC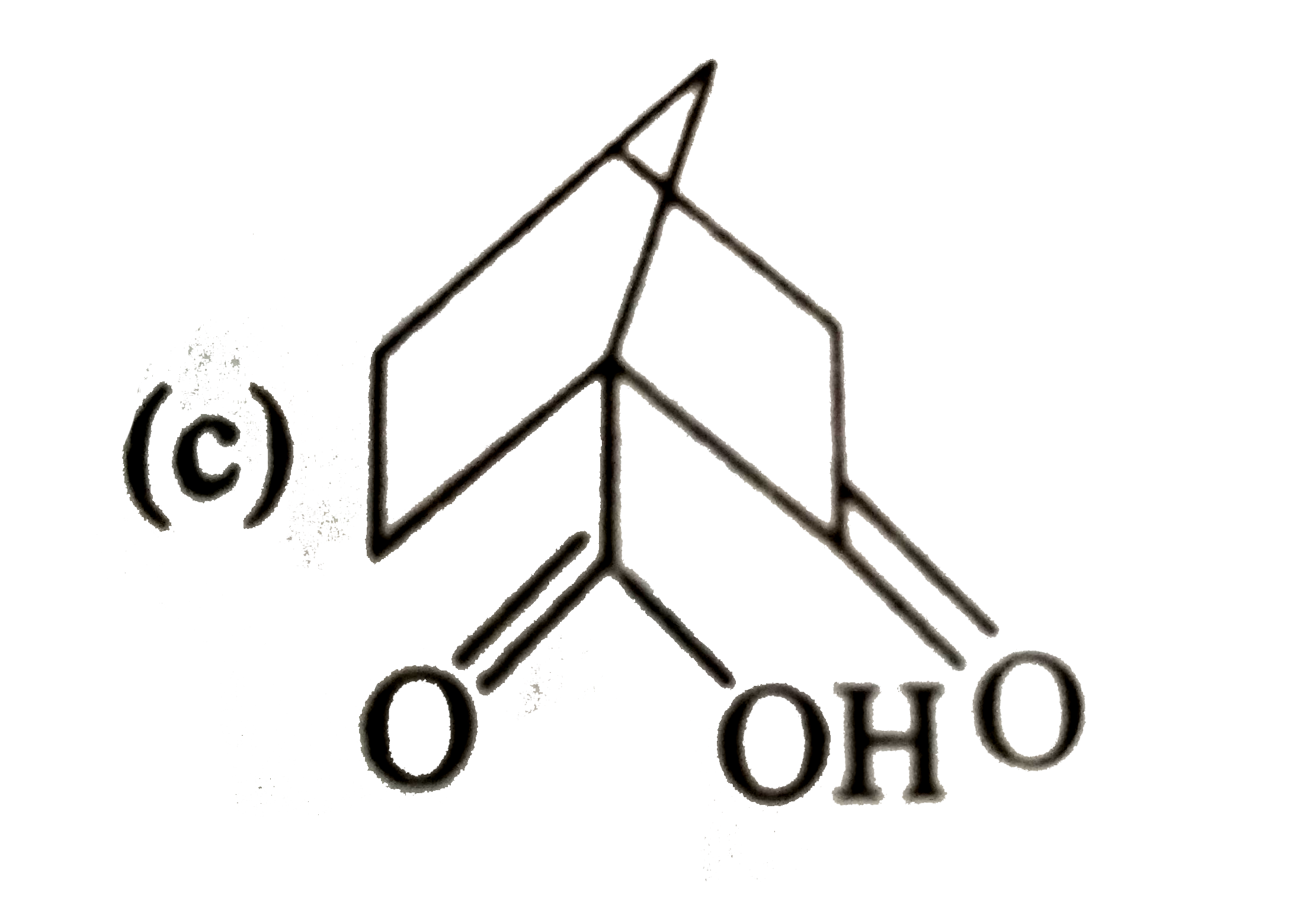 D
D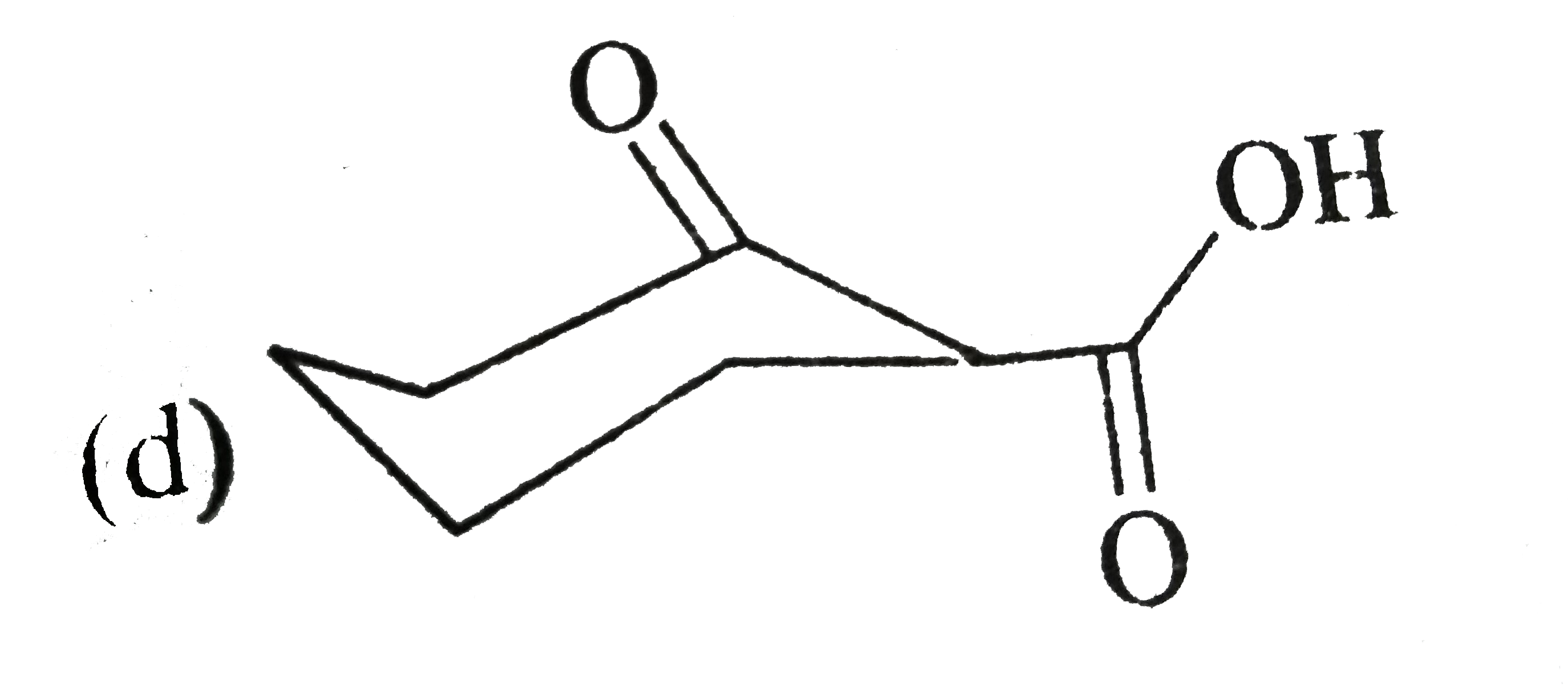
Which of the following Carboxylic acids donot undergo decarboxylation on heating?
A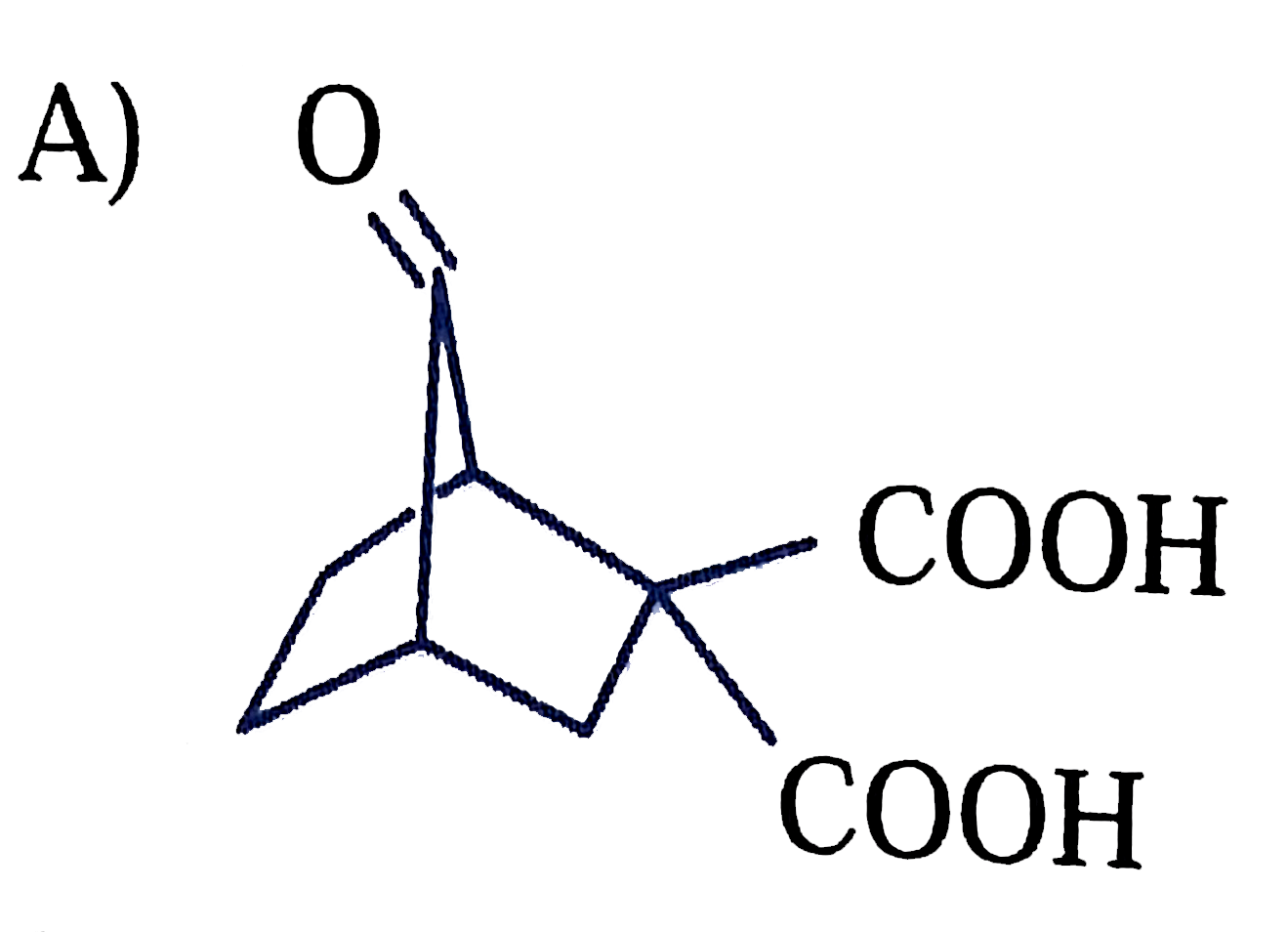 B
B C
C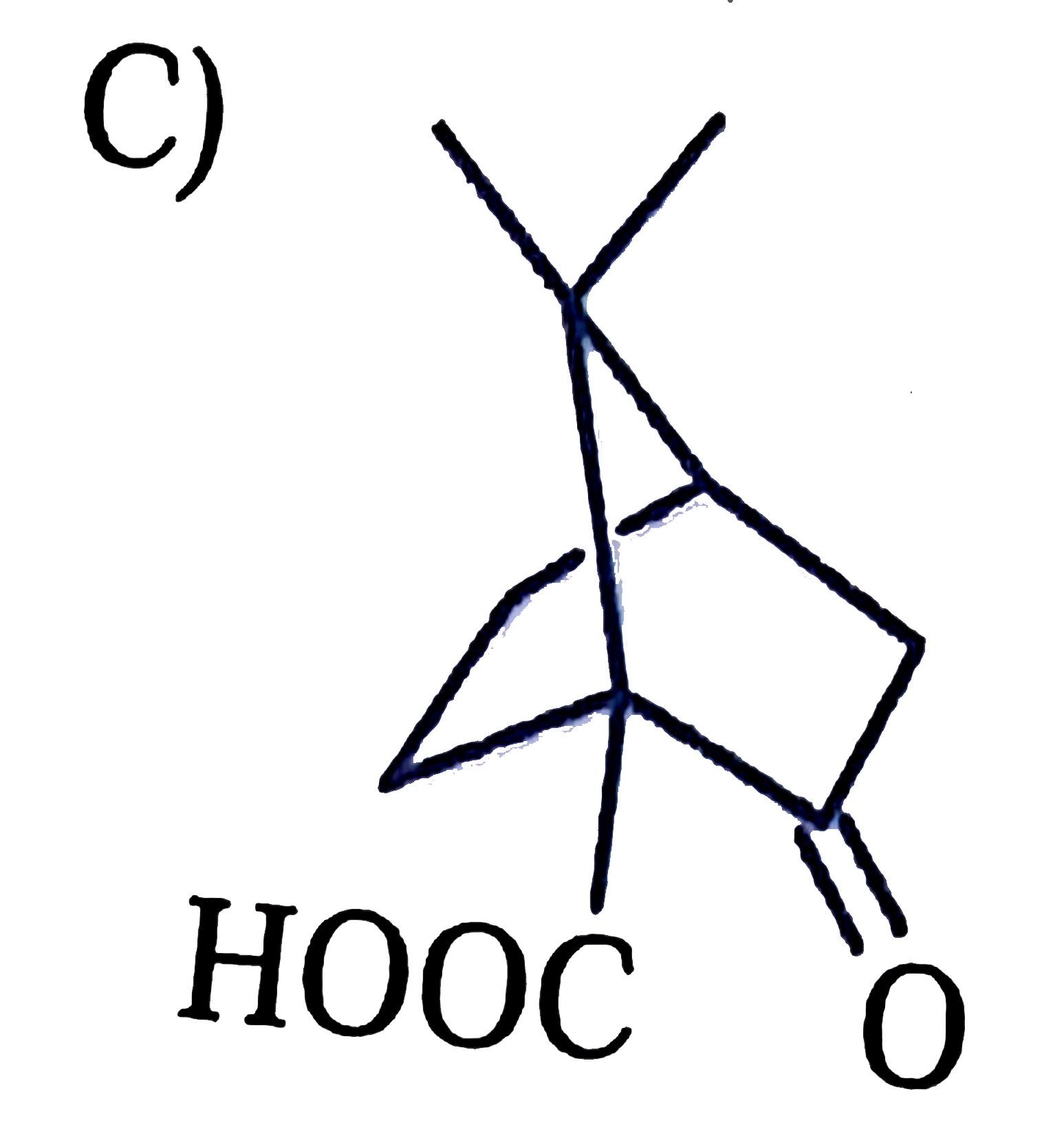 D
D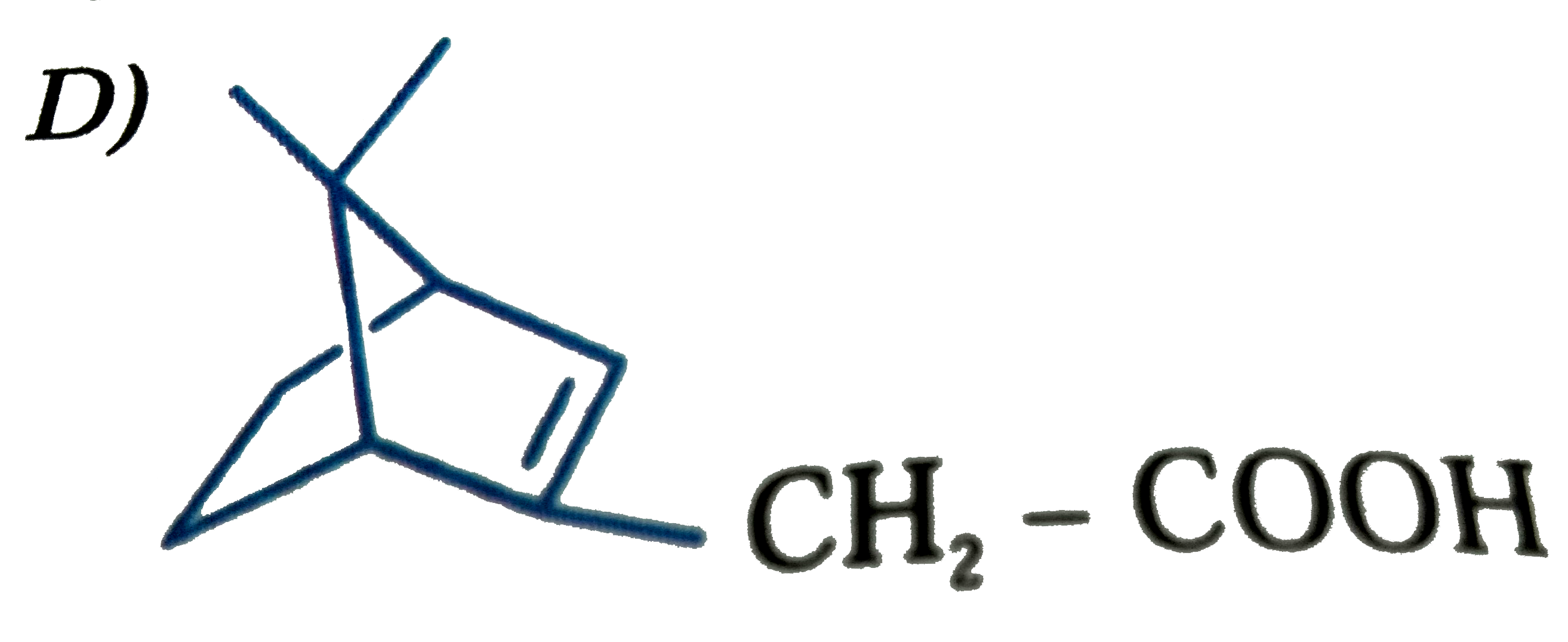
Which of the following carboxylic acid can undergo deccarboxylation on simple heating even in the absence of soda lime ?
A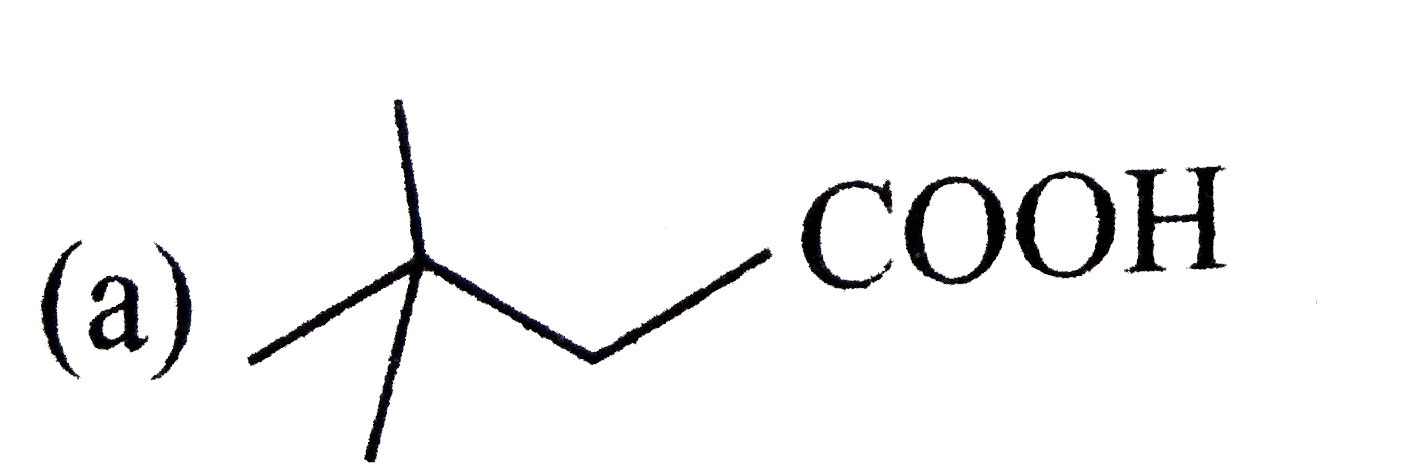 B
B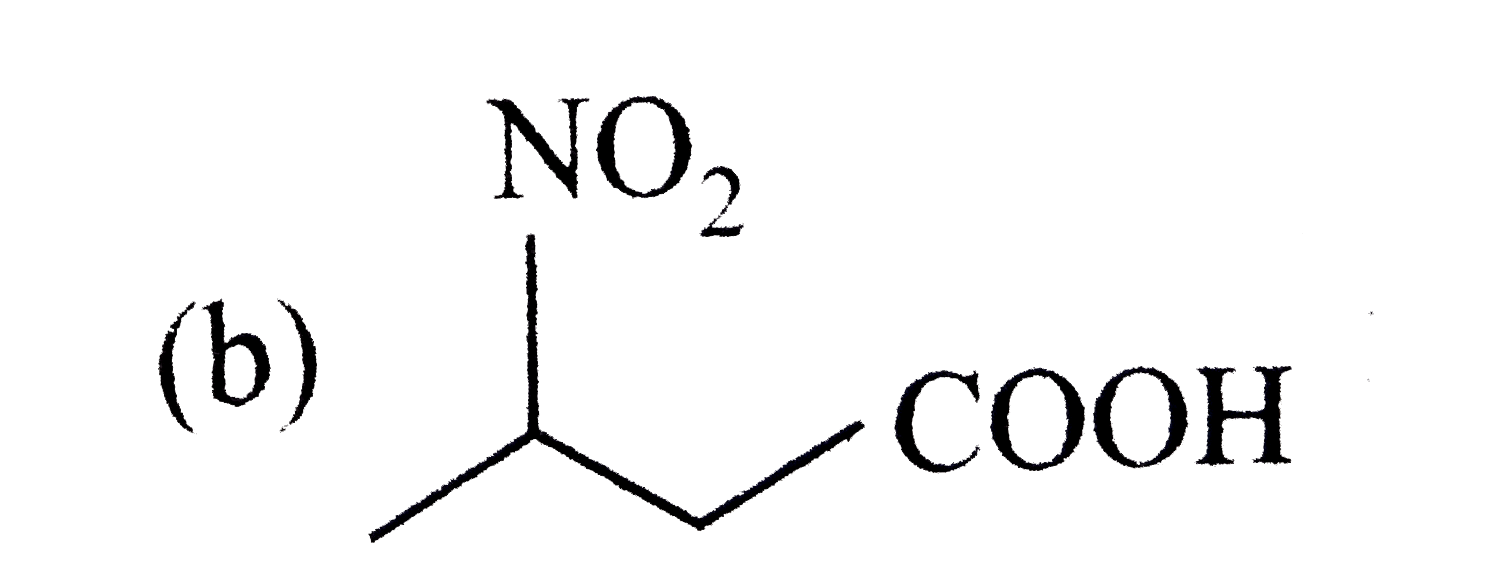 C
C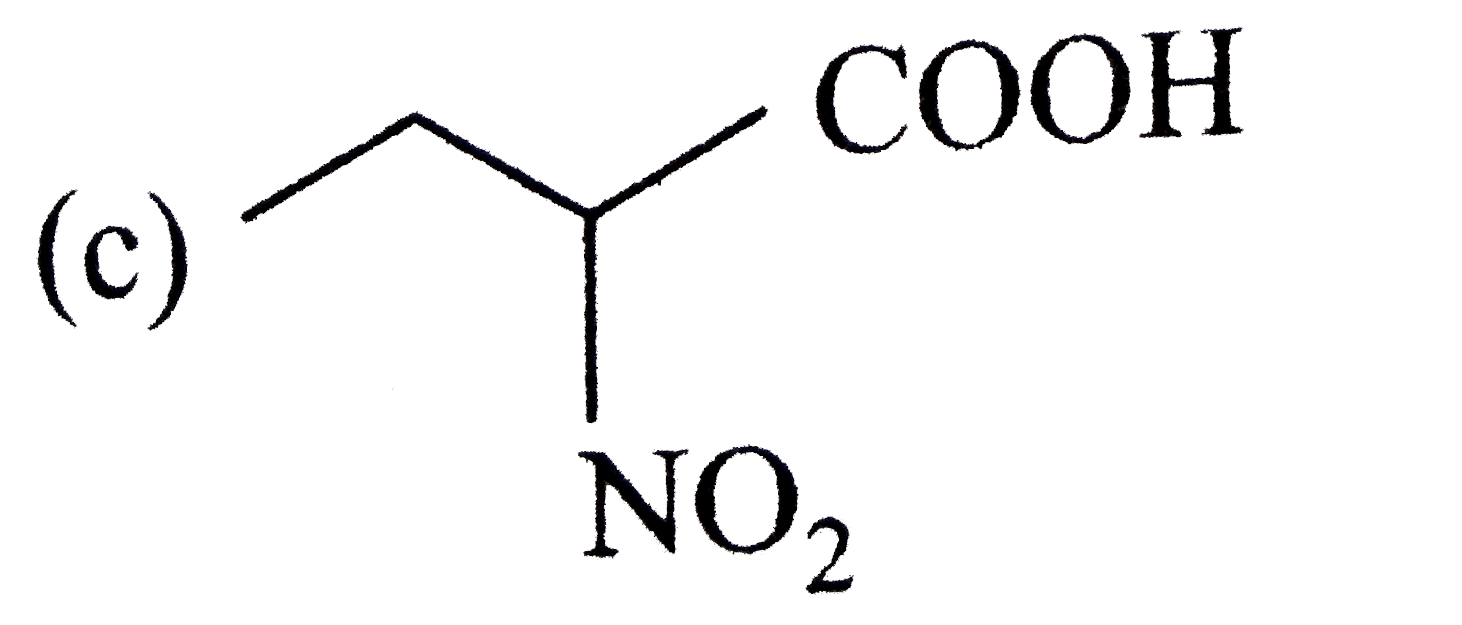 D
D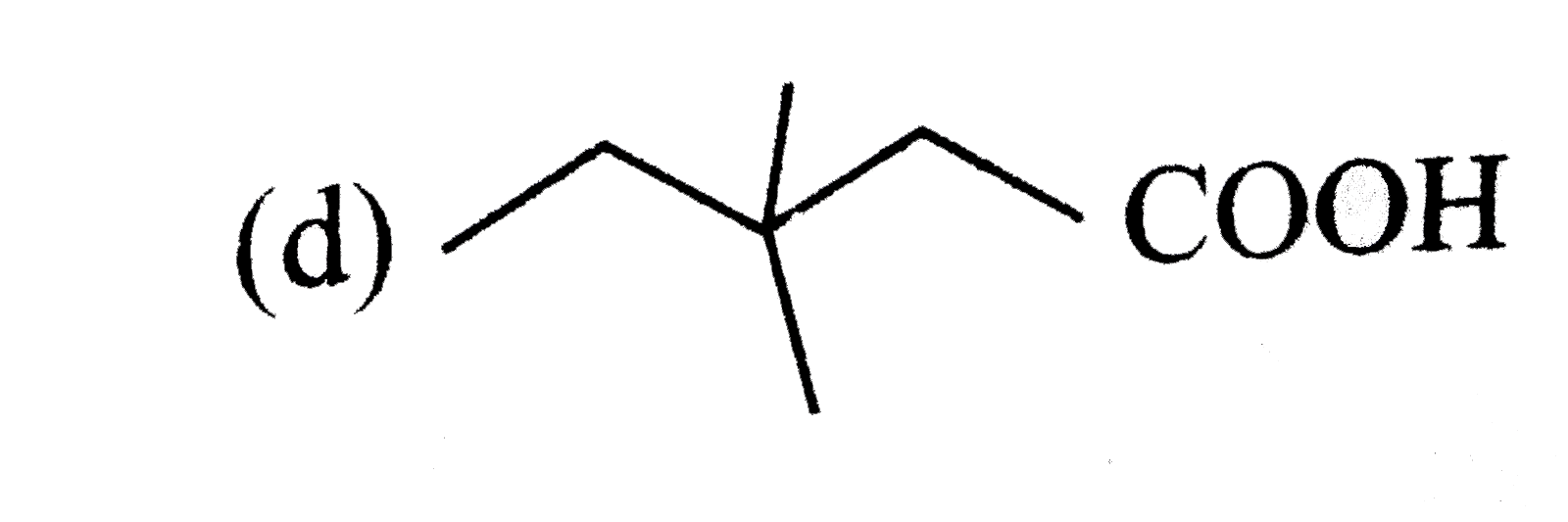
Which of the following carboxylic acids undergoes decarboxylation most easily on heating?
View SolutionWhich of the following carboxylic acids undergoes decarboxylation on heating ?
View SolutionWhich of the following carboxylic acids undergoes decarboxylation most easily ?
View SolutionWhich of the following carboxylic acids undergoes decarboxylation easily ? Explain briefly
View SolutionWhich of the following carboxylic acid is most reactive towards decarboxylation?
View Solution