For the reaction, ? 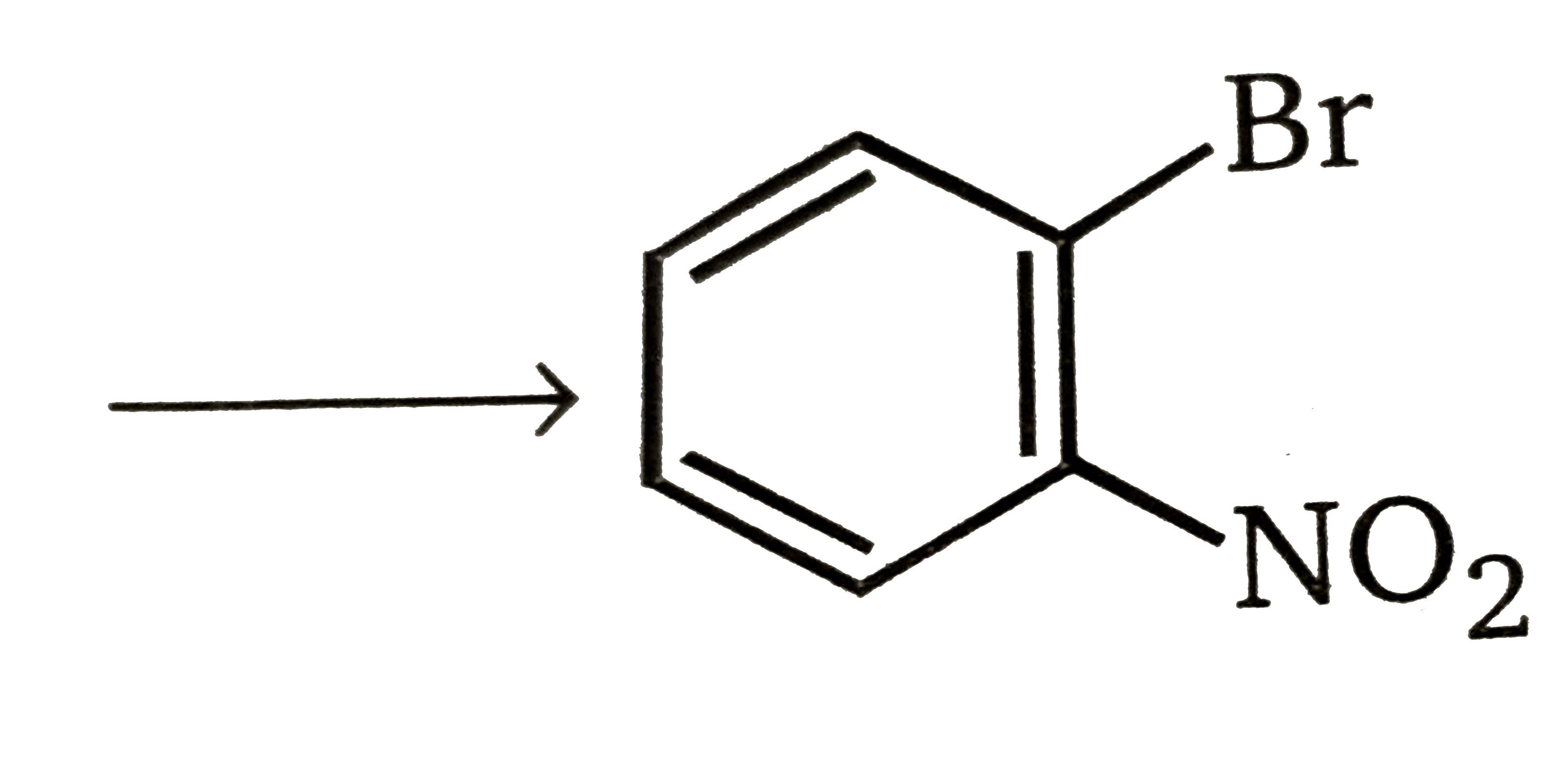 , the best combination of reactants is:
, the best combination of reactants is:
A
C6H6Br+HNO3,H2SO4
B
C6H5Br+H2SO4, heat
C
C6H5NO2+Br2,FeBr3
D
C6H5NO+HBr
Video Solution
Text Solution
The correct Answer is:A
−..B..r: is (o/pdirector)
|
Topper's Solved these Questions
- AROMATIC COMPOUNDSBook:MS CHOUHANChapter:AROMATIC COMPOUNDSExercise:Level 1 (Q.31 To Q.60)
Explore 1 Video
- AROMATIC COMPOUNDSBook:MS CHOUHANChapter:AROMATIC COMPOUNDSExercise:Level 1 (Q.121 To Q.150)
Explore 3 Videos
- AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESBook:MS CHOUHANChapter:AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESExercise:ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )
Explore 32 Videos
- BIOMOLECULESBook:MS CHOUHANChapter:BIOMOLECULESExercise:Level 2
Explore 1 Video
Similar Questions
Knowledge Check
For a first order reaction, the concentration of reactant :
Ais independent of timeBvaries linearly with timeCvaries exponentially with timeDNoneThe rate of a reaction is doubled when the concentration of reactant is doubled. What is the order of the reaction ?
AtwoBoneChalfDone and a halfFor a second order reaction, half-life concentration of reactants:
AIndependent of initial concentration of reactantsBDirectly proportional to concentration of reactantsCInversely proportional to concentration of reactantsDInversely proportional to the square of concentration of reactants
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is
(i) doubled (ii) reduced to half?
View SolutionA reaction is second order with respect to a reactant.
How is the rate of reaction affected if the concentration of the reactant is (i) doubled (ii) reduced to half ?
View SolutionWhich is the best combination of regent for the reaction
View SolutionThe rate of reaction at unit concentation of reactants is called __.
View SolutionIn a first order reaction with time the concentration of the reactant decreases
View Solution
