Nucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
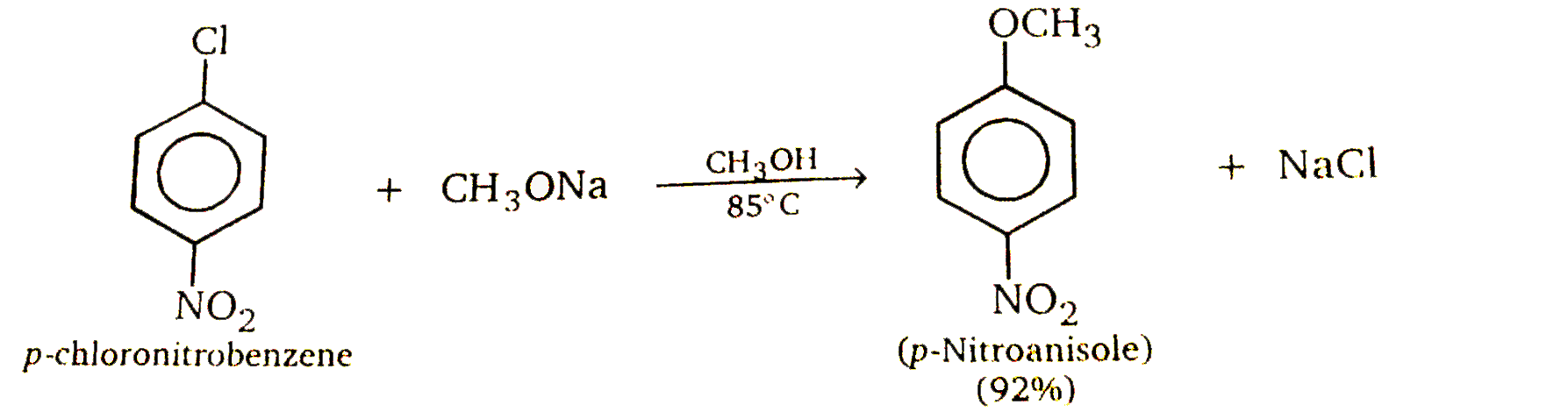
Match the column I and II
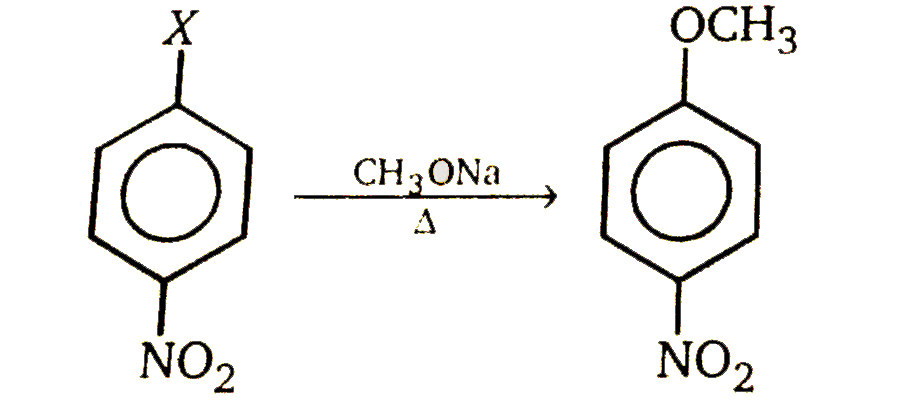
Column (I)Column (II)X=halogenrelative reactivity toward(SNAr)(a)−F(p)312(b)−Cl(q)1(c)−Br(r)0.8(d)−I(s)0.6
The correct Answer is:A-a-p, b-q, c-r, d-s
⇒ Mesenhemier complex is more stable by stron −I group.
Similar Questions
Knowledge Check
Nucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
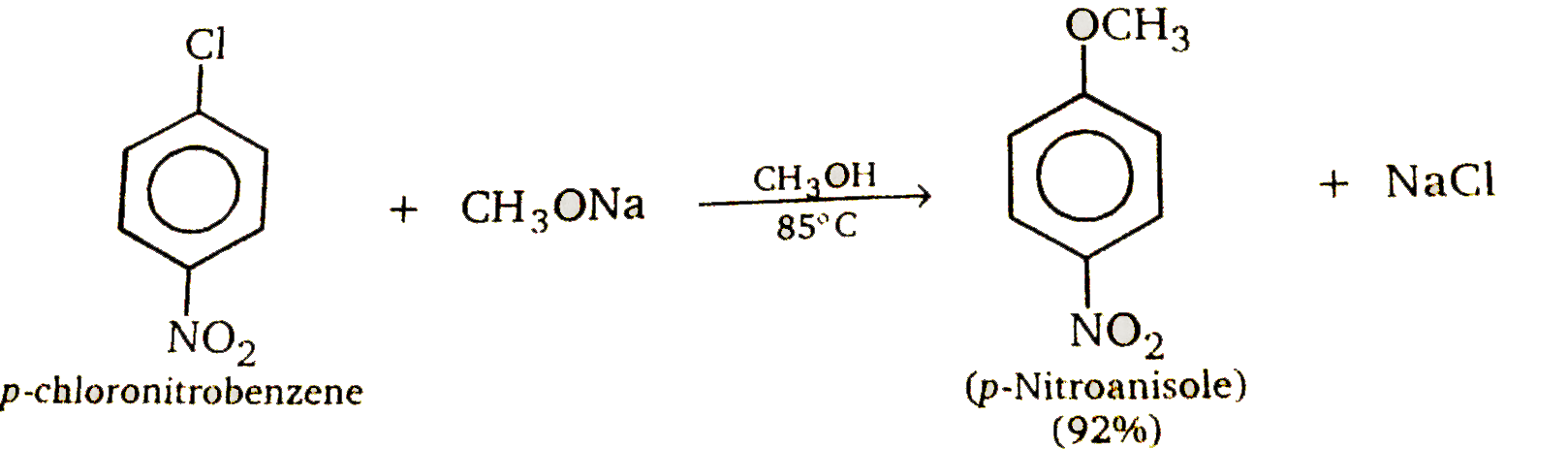
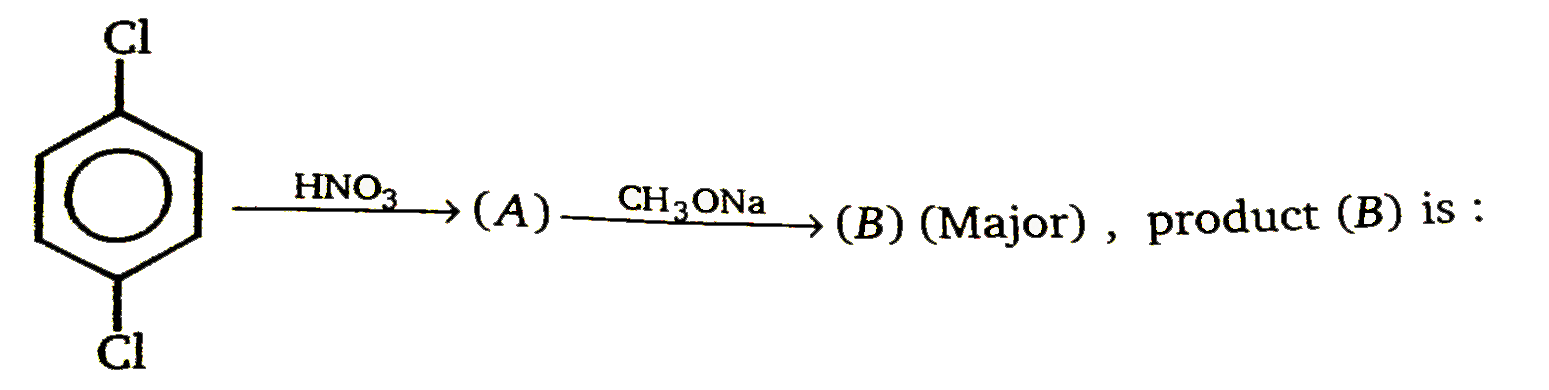 , Product (B) is:A
, Product (B) is:A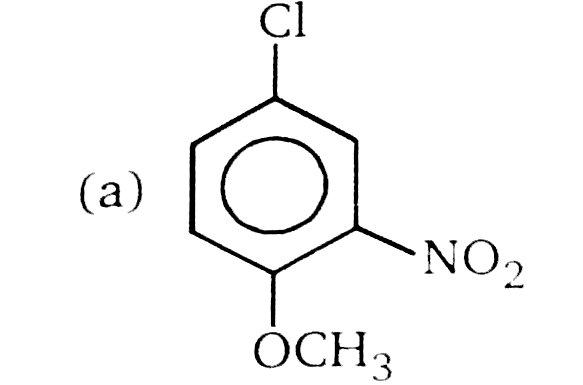 B
B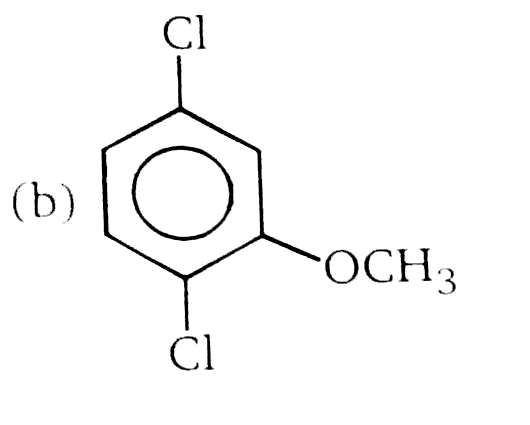 C
C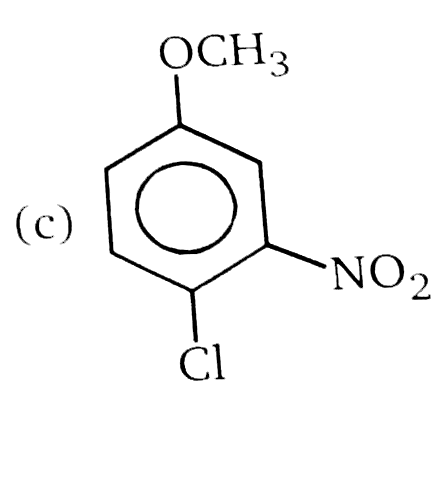 D
D
Nucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
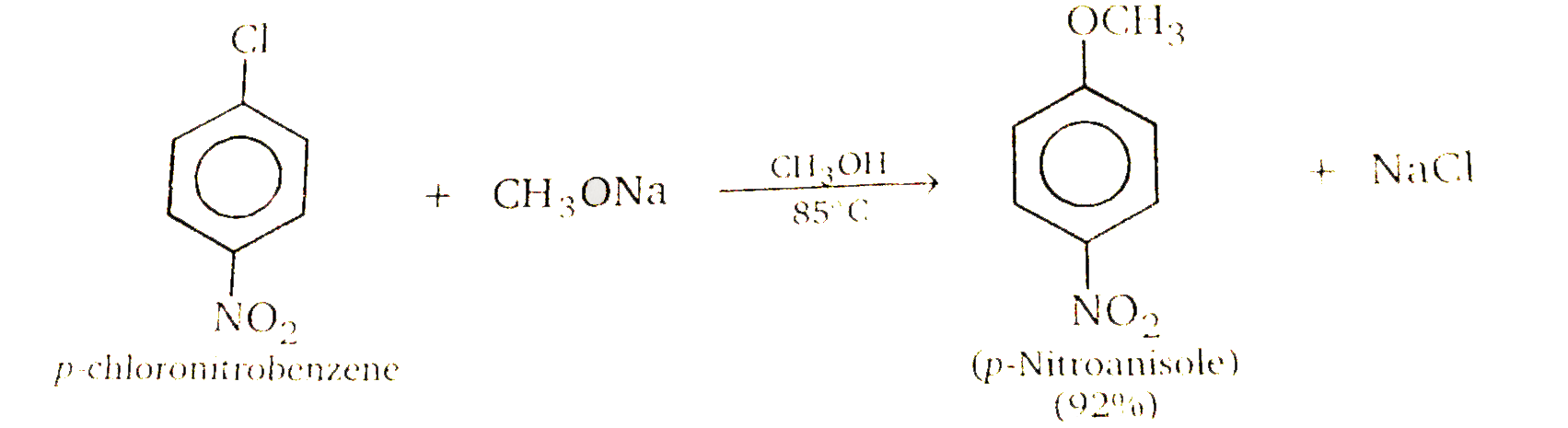
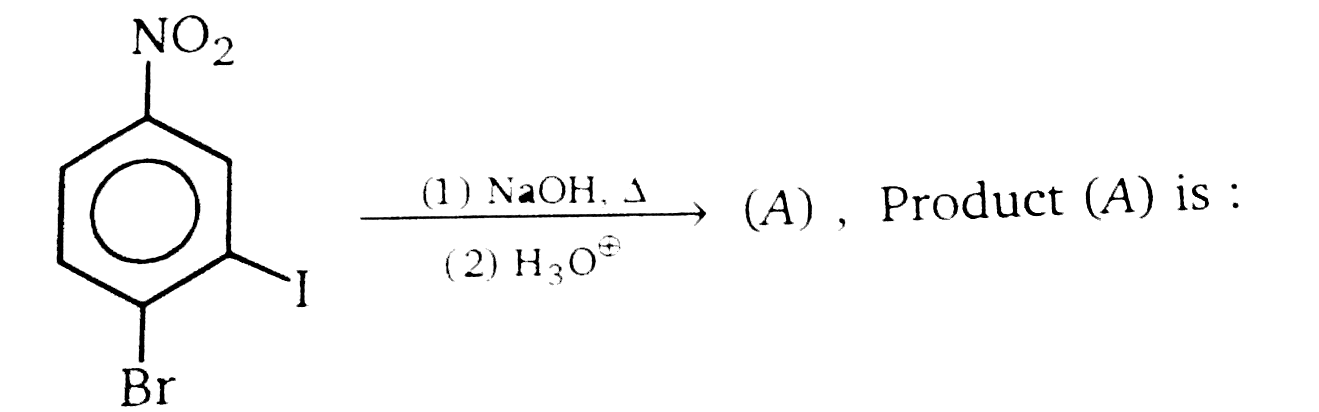 , Product (A) is:A
, Product (A) is:A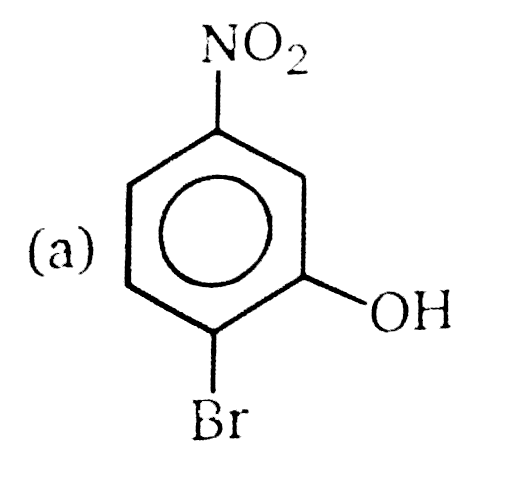 B
B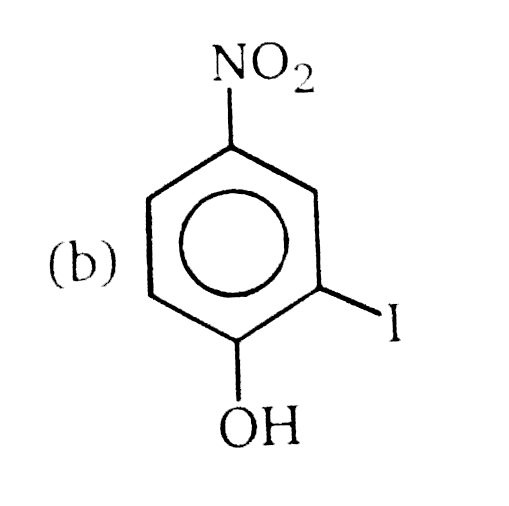 C
C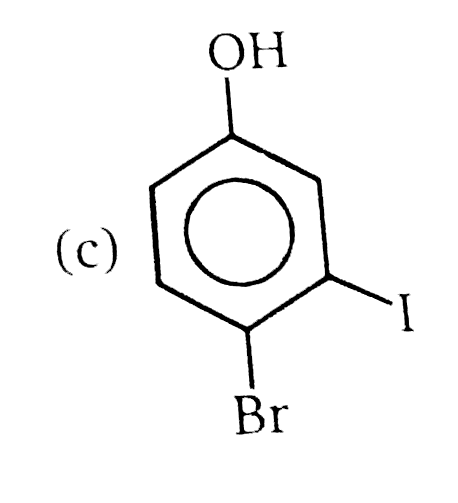 D
D
Nucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
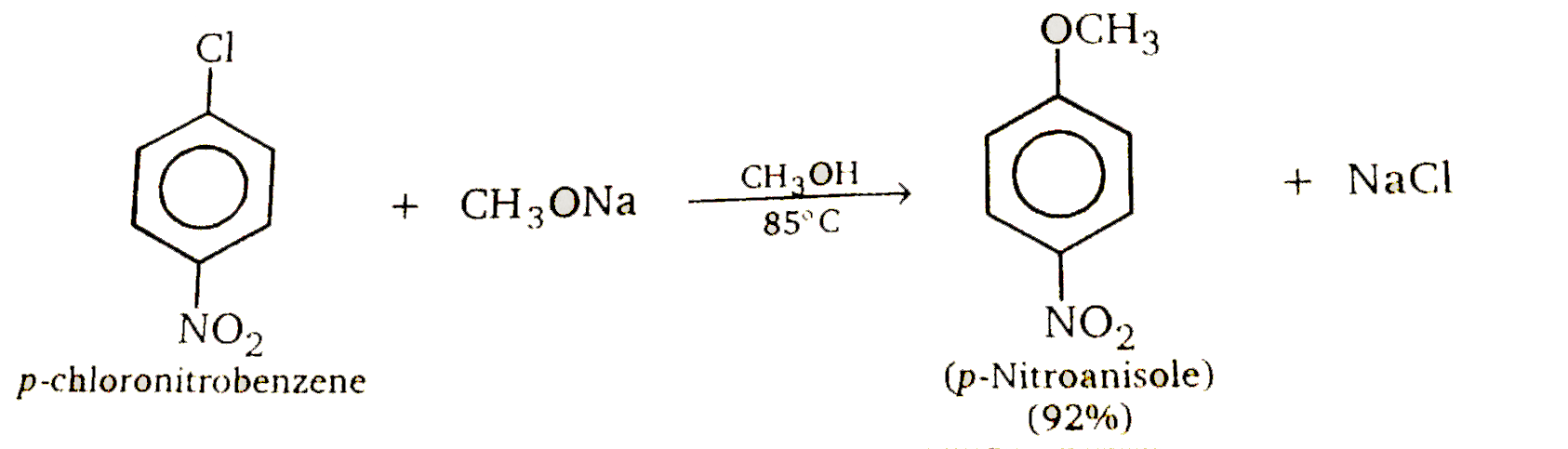
Which of the following is most reactive towards SNArA B
B C
C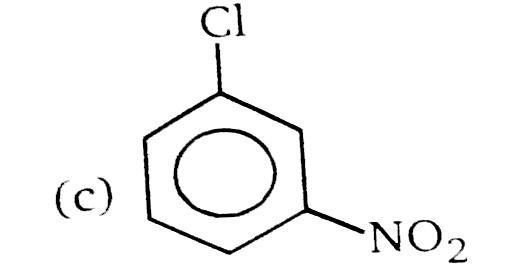 D
D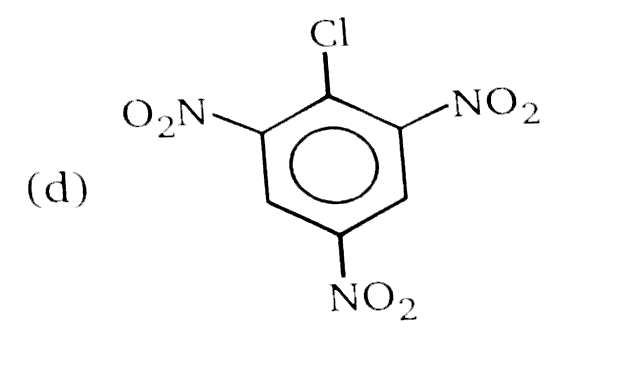
∣∣ ∣ ∣ ∣ ∣ ∣∣Column IColumn II(A)Adenine(p)Purine(B)Thymine(q)Pyrimidine(C)Uracil(r)RNA(D)Deoxyribose(s)DNA∣∣ ∣ ∣ ∣ ∣ ∣∣

View SolutionNucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
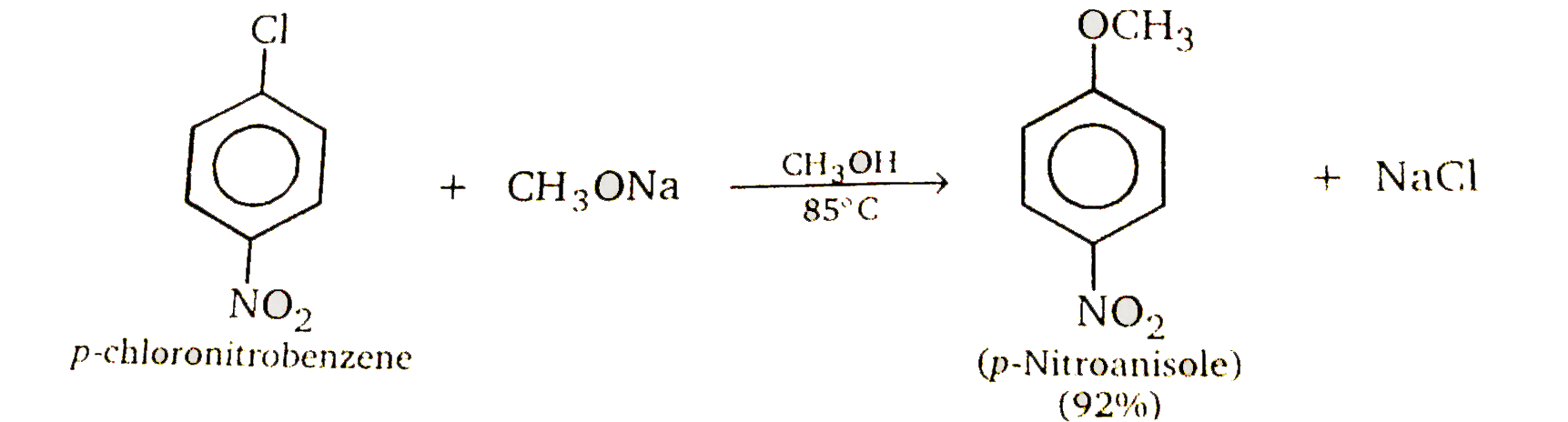
Which is the best route for the synthesis of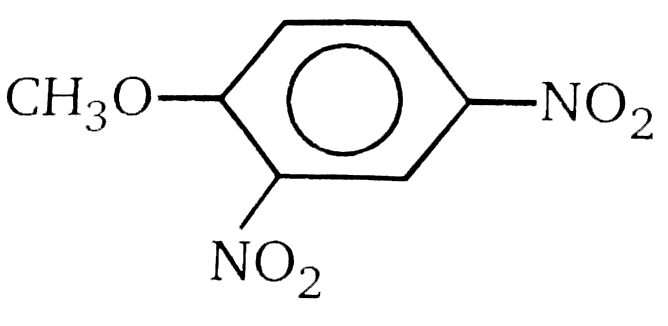 Strating from benzen of ?
Strating from benzen of ?
View SolutionNucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution
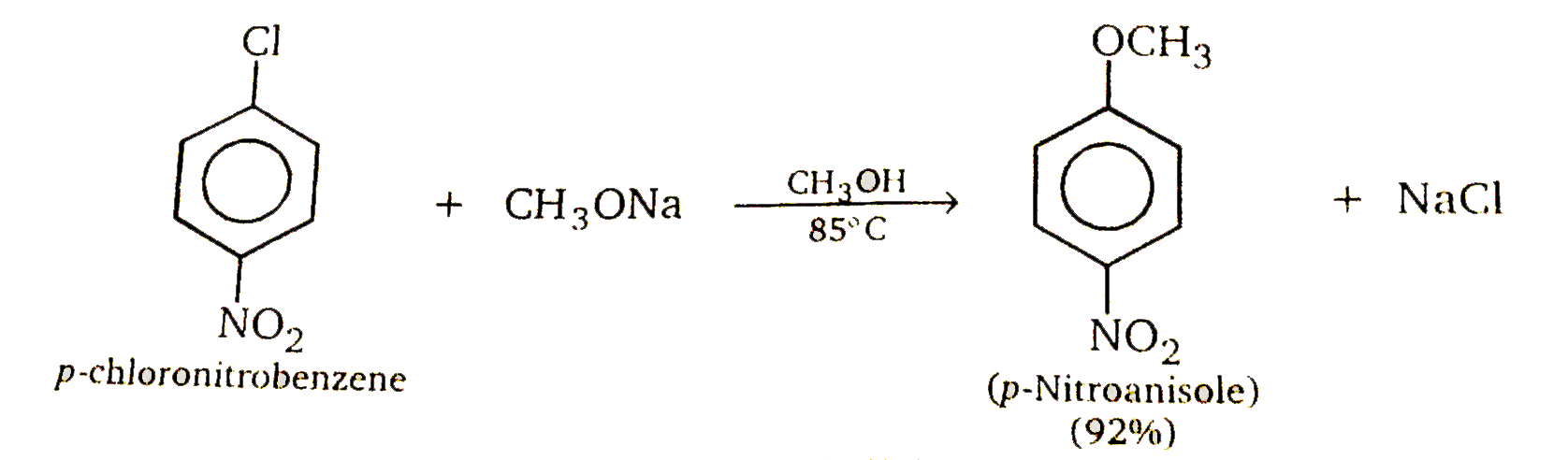
If step -2 were rate determining step, which halogen of aryl halide is most reactive towards SNAr.
View SolutionNucleophilic Aromatic substitution (SNAr):
A substituted benzene derivative containing −NO2 and Cl group at p−position is subjected to Nu− substitution

The cumulative effect of their fluorine active the rings of penta and hexa fluorobenzene toward nucleophilic aromatic substitution. What is compound X in the following synthesis ?
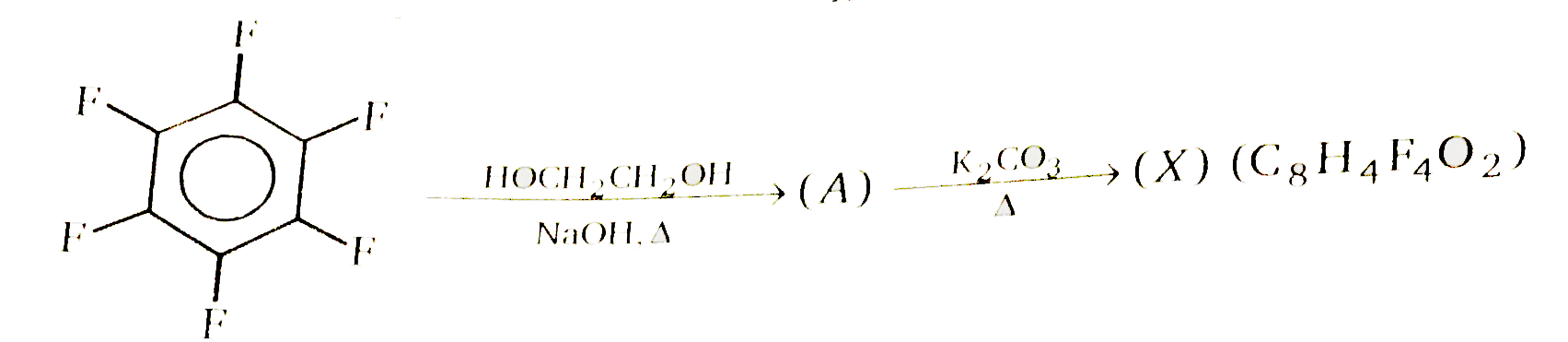
View SolutionColumn IColumn II(Metal ion configuration in weak ligand field)(CFSE, Δ0 value)(A)d4(p)0.0(B)d5(q)−0.4(C)d6(r)−0.6(D)d7(s)−0.8
View Solution

