For a given mass of an ideal gas, which of the following statements is CORRECT ?
At a constant temperature, the pressure is directly proportional to the density of al gas.
At a constant temperature, the pressure is directly proportional to the volume on a gas
At a constant pressure, the volume is inversely proportional to the temperature of a gas.
At a constant volume, pressure is inversely proportional to the temperature of a gas.
The correct Answer is:A
Step by step video, text & image solution for For a given mass of an ideal gas, which of the following statements is CORRECT ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STATES OF MATTER (GASES AND LIQUIDS) BOOK - TARGET PUBLICATIONCHAPTER - STATES OF MATTER (GASES AND LIQUIDS) EXERCISE - CRITICAL THINKING
68 Videos
- STATES OF MATTER (GASES AND LIQUIDS) BOOK - TARGET PUBLICATIONCHAPTER - STATES OF MATTER (GASES AND LIQUIDS) EXERCISE - COMPETITIVE THINKING
50 Videos
- SOME BASIC CONCEPTS OF CHEMISTRYBOOK - TARGET PUBLICATIONCHAPTER - SOME BASIC CONCEPTS OF CHEMISTRYEXERCISE - EVALUATION TEST
20 Videos
- SURFACE CHEMISTRYBOOK - TARGET PUBLICATIONCHAPTER - SURFACE CHEMISTRYEXERCISE - Evaluation Test
10 Videos
Similar Questions
Which of the following is INCORRECT regarding instantaneous dipole moment ?
View SolutionWhich one of the following statements is WRONG for gases ?
View SolutionWeight of 112ml of oxygen at NTP on liquefaction would be
View SolutionA negatively charged suspension of clay in water will need for precipitation the minimum amount of
View SolutionFor the given ideal gas equation PV=nRT, answer the following questions:
At constant temperature, in a given mass of an ideal gas
View SolutionWhich of the following options is correct w.r.t. ideal gas
View SolutionFor fixed mass of an ideal gas a constant temperature. Which is correct graph ?
View Solutionनिम्न में से कौन-सा कथन आदर्श गैस के संदर्भ में सत्य नहीं है ?
View SolutionWhich of the following statements is CORRECT for an ideal gas ?
View SolutionWhich of the following curve is correct for a given amount of an ideal gas at constant pressure?
View SolutionWhich of the following statements is NOT true for an ideal gas ?
View Solutionदिए हुए रेखाचित्र में एक आदर्श गैस के लिए रूद्धोष्म (adiabatic) और समतापी (isothermal) अवस्थाओं में उत्क्रमणीय प्रसरण (reversible expansion) दिखाया गया है। निम्नलिखित प्रकथनों में से कौन-सा कथन सही है/हैं?
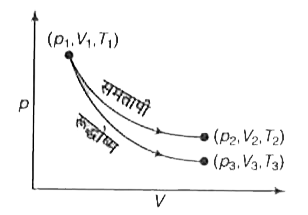
View Solutionदिये हुए रेखाचित्र में एक आदर्श गैस के लिए रूद्धोष्म (adiabatic) और समतापी (isothermal) अवस्थाओं में उत्क्रमणीय प्रसार (reversible expansion) दिखाया गया है। निम्नलिखित प्रकथनों में से कौन प्रकथन सही है/ हैं
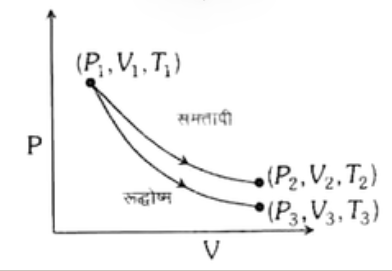
View SolutionWhich statements are correct for a process involving an ideal gas?
View SolutionWhich statements are correct for a process involving an ideal gas?
View Solution

