(i) Measurement of atomic mass of an element is very difficult? Give reason.
(ii) (i) Identify the bond betweenn H and F in HFF molecule. (ii) what properrty forms the basis of identification?Solution in Tamil
Step by step video & image solution for (i) Measurement of atomic mass of an element is very difficult? Give reason. (ii) (i) Identify the bond betweenn H and F in HFF molecule. (ii) what properrty forms the basis of identification? by Biology experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Similar Questions
Identify the bond between H and F in HF molecule.
View Solution(a) Identify the bond between H and F in HF molecule. (b) What property forms the basis of identification? (c ) How does the property vary in periods and in groups?
View Solutionकारण दीजिए :
(i) संक्रमण तत्व मिश्रधातुएं क्यों बनाते हैं?
(ii) लैन्थेनाइड संकुचन का क्या कारण है?
View SolutionExplain the following giving reasons :
(i) It is difficult to separate lanthanoid elements in pure state.
(ii) The transition elements form interstitial compounds.
View SolutionAtomic radius depends upon
(i) Number of bonds formed by the atom
(ii) Nature of the bonding
(iii) Oxidation state of the atom
View SolutionIdentify (A) to (F).
i.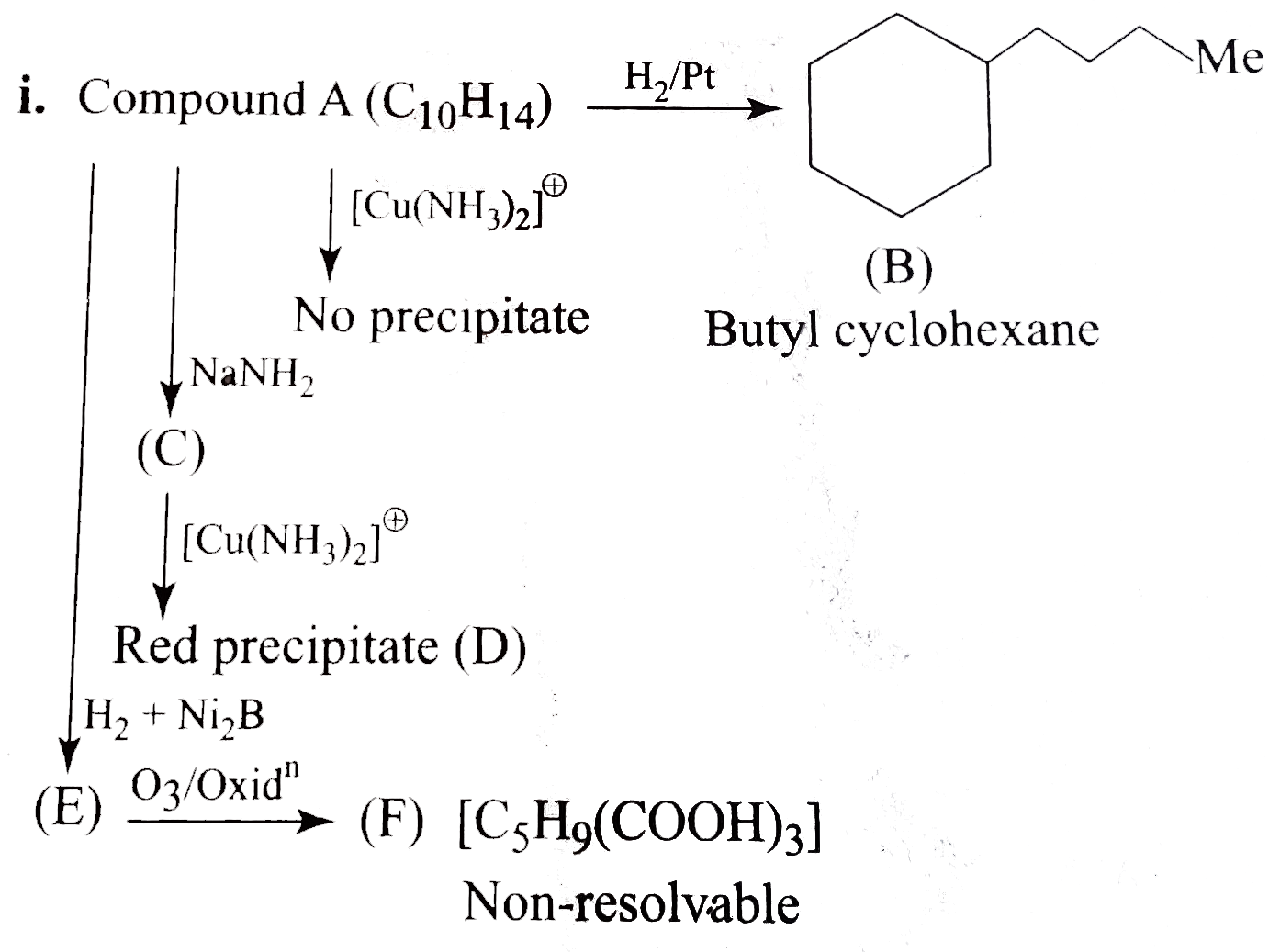
ii. What would be the structure of (A) if (F) is resolvable?
View SolutionGive reasons for the following:- i. elemental phosphorus does not exist as P_2 molecule ii. Noble gases are known as Inert gases.
View Solutionकथन I परमाणु के द्रव्यमान मापन हेतु किग्रा सुविधाजनक मात्रक नहीं है।
कथन II परमाणु का द्रव्यमान बहुत कत होता हैं
View Solution
