An organic compound on analyis found to contain 92.3 % carbon and 7.7% hydrogen. It vapour density is 39. Find its molecular formula.Solution in Kannada
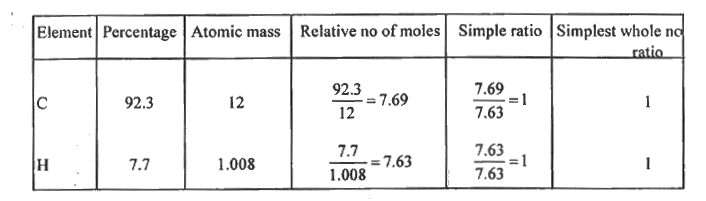
∴ Empirical formula = CH
Molecular mass = 2×39=78
∴ Molecular formula =(CH)n=(CH)6=C6H6
Similar Questions
An organic compound was found to have contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour - density was found to be 29.5. What is the molecular formula of the compound?
View SolutionAn organic compound was found to have contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour - density was found to be 29.5. What is the molecular formula of the compound?
View SolutionAn organic compound was found to have contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour - density was found to be 29.5. What is the molecular formula of the compound?
View SolutionAn organic compound was found to have contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour - density was found to be 29.5. What is the molecular formula of the compound?
View SolutionAn organic compound was found to have contained carbon = 40.65%, hydrogen = 8.55% and Nitrogen = 23.7%. Its vapour - density was found to be 29.5. What is the molecular formula of the compound?
View SolutionVapour density of a compound is 39. It contains carbon and hydrogen atoms in the ratio 1 : 1 . Its molecular formula is
View SolutionThe percentage of carbon and hydrogen in an organic compound are 92.5 and 7.5. Determine the'molecular formula of the compound if its vapour density is 39.
View SolutionAn organic compound containing carbon hydrogen and oxygen contains 52 .2 % carbon and 13.04 % hydrogen .Vapour density of the compound is 23 .Its molecular formula will be
View Solution

