Describe the structure of common from of ice.Solution in Kannada
Video Solution
Text Solution
In the normal hexagonal ice, each oxygen atom is tetrahedrally surroundanded by four other oxygen atoms, there being an hydrogen atom in between each pair of oxygen. Each hydrogen is covalent bonded to one oxygen and linked to the other oxygen by a covalent bond. Such an arrangement leads to packing having large open spaces. The density of ice is therefore less than liquid water.
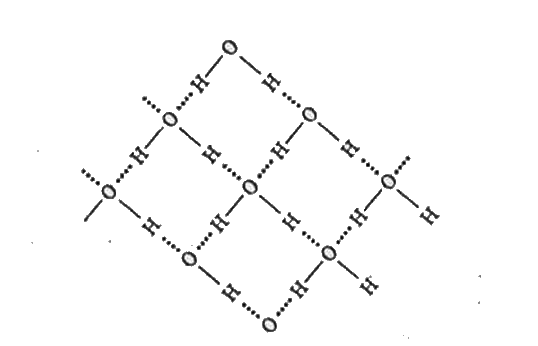
|
Similar Questions
Compare the structures of ice & water.
View SolutionExplain the structure of the common form of ice.
View SolutionWhat are the structural units of ice and dry ice?
View SolutionDescribe the structure of the common form of ice.
View SolutionDiscuss the structure of common form of ice. Why does ice float over water ?
View SolutionDescribe the structure of the common form of ice.
View SolutionDescribe the structure of the common form of ice.
View Solution
