Explain the structure of water molecule ?Solution in Kannada
Video Solution
Text Solution
A molecule of water consists of two hydrogen atoms linked to an oxygen atom through covalent bonds. He molecular dimensions of water in gas phase are shown in future (a). In the gas phase, water is a bent molecule with bond angle 104.5∘ and the O - H bond length is 95.7 pm. Water is a highly polar molecule (figure(b)) and the net dipole moment is .84 D.

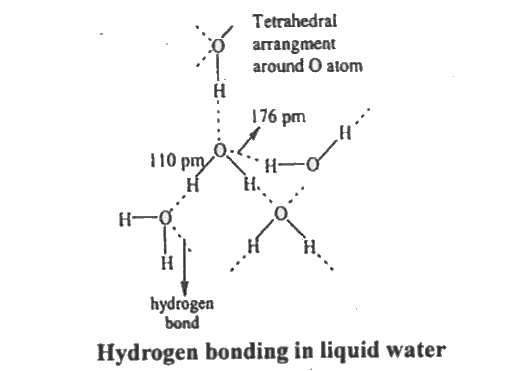
Structure of water molecule in liquid state : In liquid state, water molecules are held together by intermolecular hydrogen bonds. Each oxygen atom forms two hydrogen bonds as shown in the figure. Hydrogen bonding has a very great influence on the properties of water. An account of high dipole moment dissolves most of the ionic compounds like NaCI. Due to its ability to form hydrogen bonds water dissolves even covalent molecules like C_(2)H_(5)OH.
|
Similar Questions
The structure of water molecule is ____________
View SolutionSoul3অণুর গঠনাকৃতি ব্যাখ্যা করাে।
View SolutionExplain the structure of SO2 molecule.
View SolutionExplain the structure of ozone molecule.
View SolutionExplain the structure of CO2 molecule.
View SolutionExplain the structure of a typical antibody molecule.
View SolutionComment on the structure of water molecule
View SolutionDiscuss .the structure of water molecule.
View Solution

