Explain Sp2 hybridisation taking boron trichloride (BCl3) as an example.Solution in Kannada
Video Solution
Text Solution
Boron (5)
Electronic configuration 1−s22−s22p1
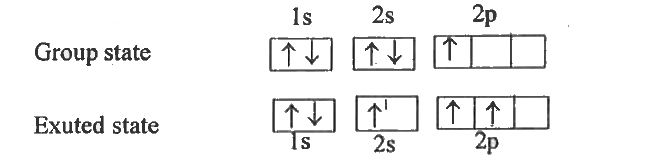
Here one 2s and two 2p half filled orbitals undergo hybridization to give three sp2 hybrid orbitals with these hybrid orbitals. The P-orbitals of chlorine molecule overlap to form π bonds.
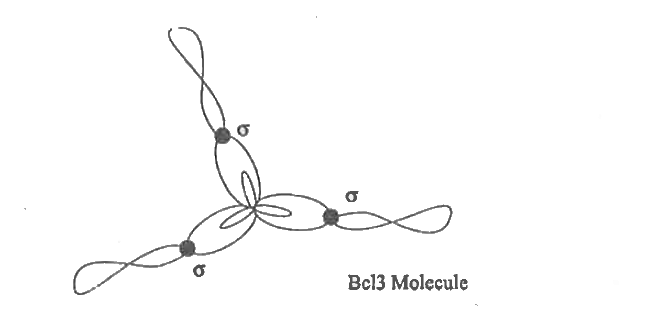
|
Similar Questions
The state of hybridisation of boron in BCl3 is
View SolutionExplain sp hybridisation with an example.
View SolutionExplain sp3 d hybridisation with a suitable example.
View SolutionExplain about sp^(3)d^(2) hybridisation with an example.
View SolutionIn BCl3, the hybridisation state of Boron is :
View SolutionExplain sp hybridisation taking acethylene as example.
View SolutionThe type of hybridisation of boron in diborane is
(a) sp , (b) sp2 , (c) sp3 , (d) dsp2
View SolutionExplain sp^3 hybridisation taking methane (CH_4) as an example
View Solution
