Balance the following redox reaction by using oxidation number method.
MnO2+Br−→Mn+2+Br2+H2O (acid medium)Solution in Kannada
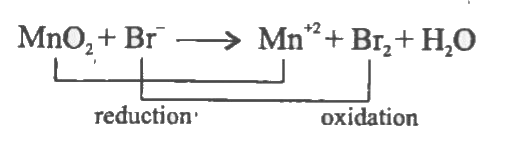
Oxidation underset(-1)(2Br^(-1))overset(2xx1"unit")(to)underset("zero")(Br_(2))]xxcancel(2)^(1)
Reduction MnO_(2) overset(1xx2"unit")(to)underset(+2)Mn^(+2)]xxcancel(2)^(1)
______________________________________
MnO2+2Br−1→Mn+2+2B2
Balancing Hydrogen and Oxygen by adding H2O molecules and H+ ions
MnO2+2Br−1+4H+→Mn+2+Br2+2H2O
Similar Questions
Balance the following chemical equation by ion electron method :
MnO−4+(COO)2−2+H+→Mn2++CO2+H2O
View SolutionBalance the Redox reaction using oxidation number method. SO_(2)+H_(2)StoS+H_(2)O
View SolutionBalance the following equation by oxidation number method.
MnO−4+Fe2+→Mn2++Fe3+ (acidic medium)
View SolutionFrom following redox reaction co-efficient of (a), (b) and ( c) are respectively ____
(a)MnO−+(b)Br−+(c)H+→Mn2++Br+H2O
View Solutionनिम्न अभिक्रिया को ऑक्सीकरण अंक विधि द्वारा संतुलित कीजिए।
MnO−4+Fe−2→Mn+2+Fe+3
View SolutionBalance the following redox reaction in acid medium using oxidation number method.
Cr2O7(2−)+Fe2+→Cr3++Fe3+
View SolutionBalance the following equations by oxidation number method. MnO_(4)^(-)+H_(2)O_(2)+H^(+)toMn^(2+)+H_(2)O+O_(2)
View SolutionBalance the following equations by oxidation number method. MnO_(2)+HCl toMnCl_(2)+Cl_(2)+H_(2)O
View Solution
