Explain with diagram the construction, cell reactions and symbolic representation of the Daniel cell.Solution in Kannada
Video Solution
Text Solution
Daniel cell is a electrochemical cell It consists of two half cells or two electode systems.
Zinc rod dipped in 1M ZuSO4 forms Anode. In this electrode, Zin: rod is -vely charged with respect to the solution and zinc undergoes oxidation.
Copper red dropped in 1M CuSO4 forms Cathode. In the electode, copper rod is +vely charged with respect lo the solution and copper ions undergoes reduction.
The metallie rods in the vessel are connected through ammeter by means of insulated wire. The solutions in the two beakers are connected by means of Salt bridge.
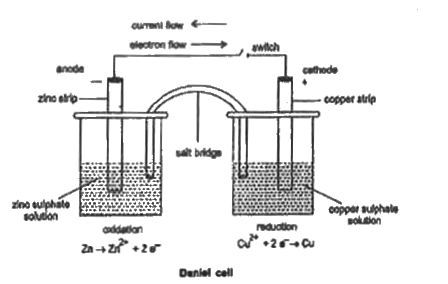
when the circuit is completed electrons flows zinc rod (anode ) to copper rod ( cathode ) in the external circuit , therefore , curtent flows from copper to zinc
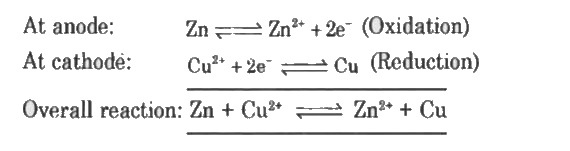
|
Similar Questions
Explain construction of the galvanic cell according to Daniell cell. I galvanic cell explain positive and negative electrode with suitable chemical reaction.
View SolutionExplain symbolic representation of galvanic cell (electrochemical cell) by suitable example.
View SolutionGive symbolic representation of Daniel cell.
View SolutionWhat is the representation of a Daniell cell ?
View SolutionExplain in detail the symbolic representation of electrochemical cells.
View Solutioncell reaction in Daniel cell is a ___ process.
View SolutionSalt Bridge | Construction of Galvanic Cell | Representation of a Cell
View SolutionDaniel Cell | Salt Bridge | Construction of Galvanic Cell | Representation of a Cell | Nerst Equation Based questions
View Solution
