What is the role of lime stone in the extraction of iron from the concentrated hematite ore ? Solution in Kannada
Video Solution
Text Solution
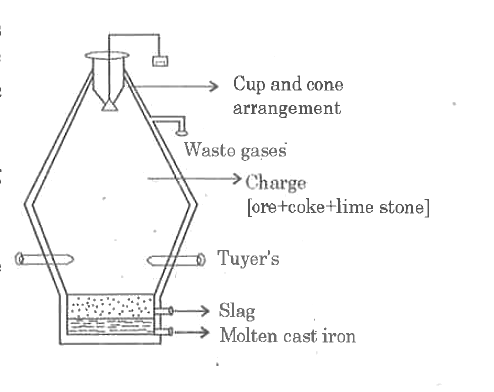
Concentration ore is mixed with lime stone and coke, and fed into blast furnace. Coke acts as fuel, CO formed from it acts as reducing agent and lime stone acts as flux.
Reactions in the blast furnance.
(i) Zone of combination : Hot air is blown from the bottom of the furnance coke burns to give CO2 temperature rises to 2200 K.
C+O2→CO2
The CO2 reacts wwith overlying coke to from CO.
CO2+C→2CO.
(ii) Zone of reduction : In the upper part of the furnance where the temperature range is 500 K to 800 K CO reduces iron oxides to iron.
Fe2O3+3CO→2Fe+3CO2
(iii) Zone of slag formation : In the middle part of the furnance where the temperature range is 800 K to 1000 K limestone decomposes to CaO, which removes silicate impurity to form slag.
CaCO3→CaO+CO2
CaO+SiO2→CaSiO3.
|
Similar Questions
What is the role of lime stone and coke in the extraction of iron using blast furnace?
View Solutionप्रक्कथन : आयरन के निष्कर्षण के लिए हैमेटाइट अयस्क प्रयुक्त होता है
कारण : हैमेटाइट आयरन का कार्बोनेट अयस्क है
View SolutionWhat is the role of lime is the extraction of iron from red Haematite?
View SolutionWhat is the role of lime is the extraction of iron from red Haematite?
View SolutionDuring extraction of iron, what is the role of lime stone ?
View SolutionWhat is the role of lime stane in the extraction of iron ?
View SolutionWhat is the role of lime stone in the extraction of iron ?
View Solutionहेमेटाइट से लोहा प्राप्त करने में चूने का पत्थर डालते हैं, यह कार्य करता है
View Solution
