How ammonia is manufactured by Haber's process ? Solution in Kannada
Video Solution
Text Solution
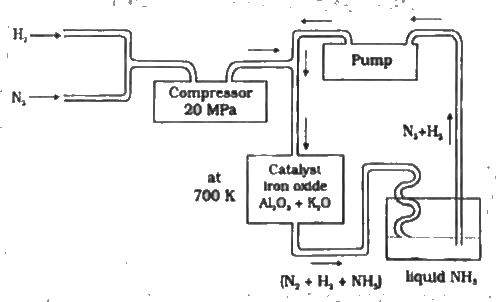
Ammonia is manufactured by Haber's process by the direct combination of nitrogen and hydrogen.
N2(g)+3H2(g)→2NH3(g)
According to Le- chatelier 's principle yield of ammonia is increased by
(i) High pressure of 200 atm.
(ii) Optimum temperature of 700 atm.
Use of catalyst like iron oxide with K2OandAl2O3
|
Similar Questions
Name the promoter used in the Haber's process for the manufacture of ammonia.
View SolutionExplain the principles involved in the manufacture of ammonia by Haber's process.
View SolutionThe catalyst used in the manufacture of ammonia by Haber's process is
View SolutionThe catalyst used in the manufacture of ammonia by Haber's process is
View SolutionIn the Haber's process for the manufacture of ammonia the catalyst used is
View SolutionWhich catalyst is used in the manufacture of ammonia by Haber's process ?
View Solution……..is used in manufacture of ammonia by Haber's process
View SolutionDescribe the manufacture of ammonia by Haber's process with favourable conditions.
View Solution

