Which is the strongest acid among the hydrogen halides? Give one reason
[X=F,Cl,Br,I]Solution in Kannada
Video Solution
Text Solution
Hl is the strongest acid among the hydrogen halides because it has low bond dissociation enthalpy.
|
Similar Questions
The covalent alkaline earth metal halide ( X = Cl, Br, I ) is :
View SolutionIdentify the halogen (F, Cl, Br or I).
which is the strongest oxidising agent,
View SolutionIdentify the halogen (F, Cl, Br or I) which forms a hydrogen halide which is a low boiling liquid.
View SolutionWhich one among the methyl halides (CH_(3)X,X = F, CI, Br, I) possesses the highest dipole moment?
View SolutionIn the reaction :
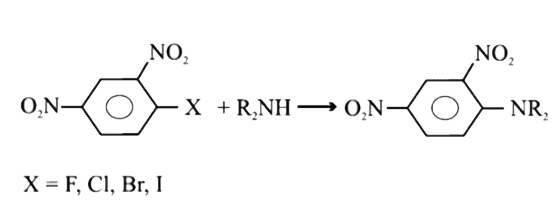
X=F,Cl,Br,I
Then which of the following aryl halide is most reactive?
View SolutionWhich is the strongest acid among the hydrogen halides? Give one reason
[X=F,Cl,Br,I]
View SolutionAssertion (A) : Among the hydrogen halides HF is weakest acid.
Reason R: Among hydrogen halides HF bond length is least.
View SolutionWhich of the following bonds is the strongest? F−F, Cl−Cl, I−I, Br−Br.
View Solution
