A––4KOH,O2−−−−−→2B––+2H2O(Green)
3B––4HCl−−→2C––+MnO2+(Puple)2H2O
2C––H2O,KI−−−−→2A––+2KOH+D––
In the above sequence of reaction A––andD–– respectively, are:
A
KIandKMnO4
B
MnO2andKIO3
C
KIO3andMnO2
D
KIandK2MnO4
Video Solution
Text Solution
The correct Answer is:B
MnO2(A)4KOHO2−−−−−→2K2MnO4(Green)(B)+2H2O
2K2MnO4(B)+4HCl→2MnO2(A)+2KOH+KIO3
2KMnO4(C)+H2O+KI→2MnO2(A)+2KOH+KIO3
|
Similar Questions
Determine the oxidation number of the underlined elements in the following
Si–––H4,B––H3,S2–––O2−3,HP––O2−4,Fe–––O4,K––2O
View SolutionWhat is the oxidation number of the underlined elements?
a. H2S–– , b. H2S––O4 c. NaS2–––O
d. Na2S4–––O6 e. Ca(HS––O3)2 f. H2S2–––O8
g. H2S––O5 , h.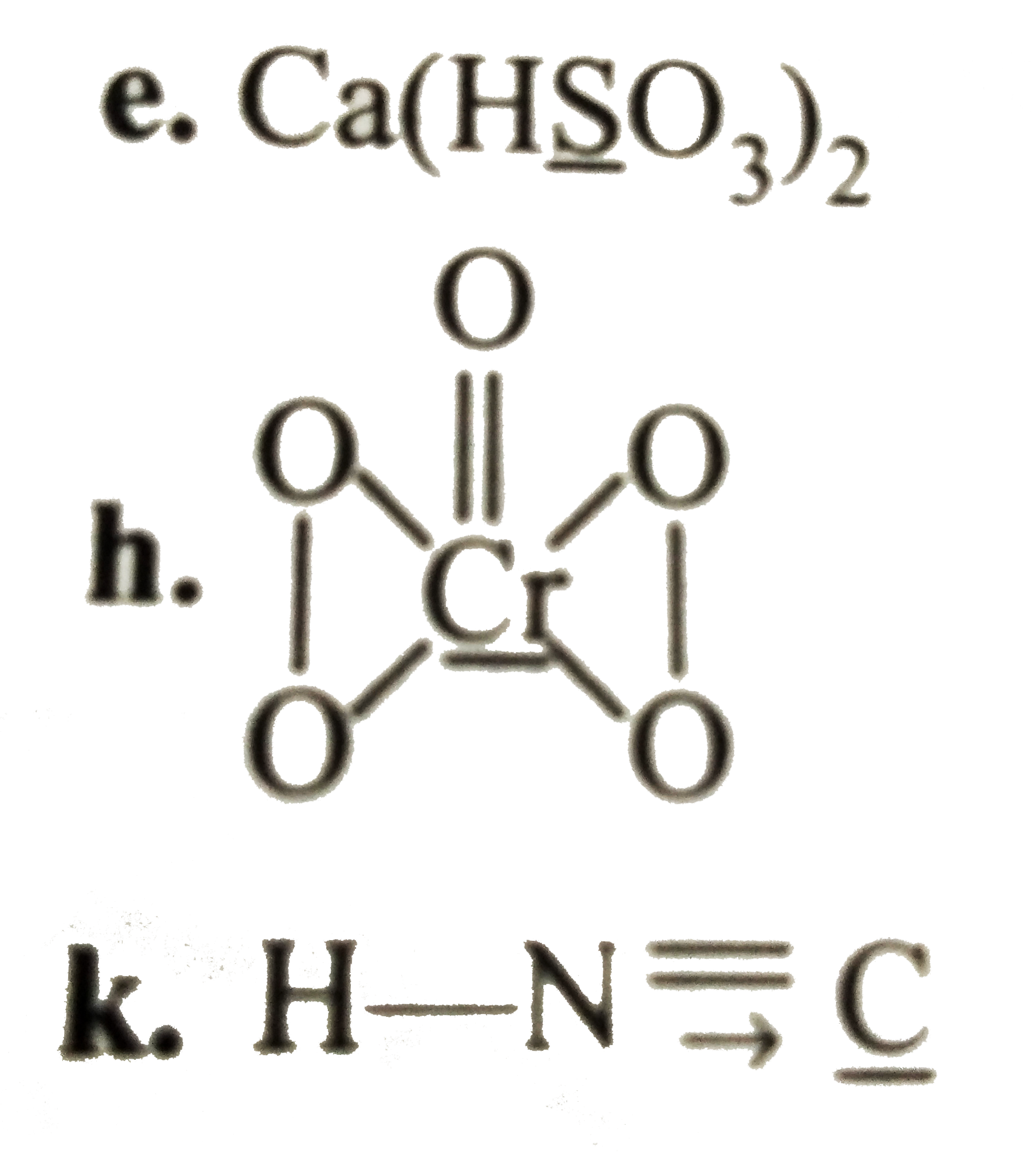 , i. N––H4N––O3 , j. H−C––≡N , k. H−N=−−→C–– , l. HN––O4
, i. N––H4N––O3 , j. H−C––≡N , k. H−N=−−→C–– , l. HN––O4
View Solution
Knowledge Check
A––4KOH,O2−−−−−→2B––+2H2O
3B––4HCl2C––(Purple)+MnO2+2H2O
2C––H2O,Kl−−−−→2A––+2KOH+D––
In the above sequence of reactions,
A––andD––, respectively, are:AKlO3andMnO2BMnO2andKlO3CKlandK2MnO4DKlandKMnO4B––rF+H2O→HBrO+HF
AIf product is oxy acid with -ic suffix.BIf product is oxy acid with -ous suffixCIf product are two oxy acids one with -ic suffix and otherone with -ous suffix.DIf product is not oxy acid, neither with -ic suffix nor with -ous suffixB––rF+H2O→HBrO+HF
AIf product is oxy acid with -ic suffix.BIf product is oxy acid with -ous suffixCIf product are two oxy acids one with -ic suffix and otherone with -ous suffix.DIf product is not oxy acid, neither with -ic suffix nor with -ous suffix
