The gas phase reaction PCl5(g)⇔PCl3(g)+Cl2(g) is an endothermic reaction. The formation of PCl5 in equilibrium mixture of PCl5(g),PCl3(g)andCl2(g) can be decreased by :
A
Decreasing the temperature
B
Addition of an inert gas at constant pressure
C
Decreasing the volume
D
Addition of an inert gas at constant volume
Video Solution
Text Solution
The correct Answer is:B
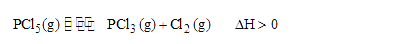
→ By decreasing the temperature, reaction will proceed in backward direction as per Le-Chatelier’s principle.
→ By adding inert gas at constant pressure, volume will increase, so Crowding will decrease so the reaction will proceed in that direction in which the crowding is more i.e. forward direction.
→ When the volume is decreased, crowding increases due to which reaction proceeds backward as per Le – Chatelier’s principle.
→ no effect.
|
Similar Questions
Knowledge Check
For the reaction, PCl5(g)→PCl3(g)+Cl2(g)
AΔH=ΔEBΔH>ΔECΔH<ΔEDnone of theseFor the reaction : PCl5(g)→PCl3(g)+Cl2(g):
AΔH=ΔUBΔH>ΔUCΔ<ΔUDNone of the aboveFor the reaction Pcl5(g)⇔PCl3(g)+Cl2(g)
AKp=KcBKp=Kc(RT)−1CKp=Kc(RT)DKp=Kc(RT)2
For the gas phase reaction
PCl5→PCl3(g)+Cl2(g)
which of the following conditions are correct?
View SolutionFor the gas phase reaction,
PCl5(g)→PCl3(g)+CL2(g)
Which of the following conditions are correct?
View SolutionFor the gas-phase decomposition,
PCl5(g)⇔PCl3(g)+Cl2(g)
View SolutionThe equilibrium : PCl5(g)⇔PCl3(g)+Cl2(g), may be shifted to the right if
View SolutionFor the reaction PCl5(g)⇔PCl3(g)+Cl2(g), the equation connecting the degree of dissociation (α) of PCl5(g) with the equilibrium constant Kp is
View Solution
