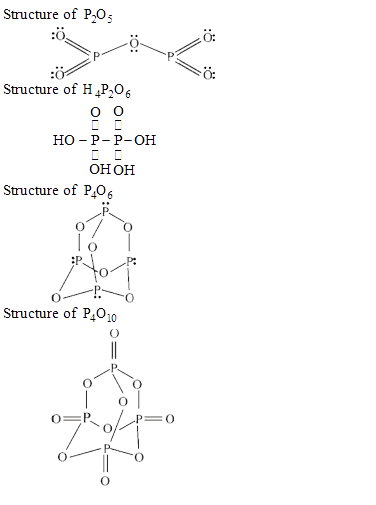
Among the following compounds of phosphorus:
H4P2O6,P2O5,P4O6,P4O10.
The compounds having P-O-P bond is(are):
All except H4P2O6
Only P4O10,P4O6,H4P2O6
Only P2O5,P4O10,H4P2O6
All four compounds
The correct Answer is:A
Similar Questions
In H_(6)P_(6)O_(18),P_(4)O_(10) how many P-O-P bonds are present respectively?
View SolutionOxidation states of P in H4P2O5,H4P2O6,H4P2O7 are respectively ________
View SolutionIn P4O6andP4O10, the number of oxygen atoms bonded to each phosphorus atoms are reppectively-
View SolutionThe equilibrium constant for the reaction? P_(4(s)) +5 O_(2(g)) rarr P_(4)O_(10(s)) (A) k_(p) =(1)/ (p_(o_(2))^(5)) (B) k_(p) = p_(o_(2))^(5) (C) k_(p) = (P_(P_(4)o_(10))) / (p_(p_(4)) p_(o_(2))^(5)) (D) k_(p) = (p_(p_(4)) * p_(o_(2))^(5)) / (P_(p_(4)o_(10))) ]
View SolutionP_(4)O_(6) and P_(4)O_(10) are formed by burning P_(4) with O_(2) as: P_(4) + 3O_(2) to P_(4)O_(6) P_(4) + 5O_(2) to P_(4)O_(10) What are the masses of P_(4)O_(6) and P_(4)O_(10) that will be produced by the combustion of 2.0 g of P_(4) in 2.0 g of oxygen leaving no P_(4) and O_(2) ?
View SolutionOxidation state of P in H4P2O5,H4P2O6,H4P2O7 are respectively
View SolutionOxidation states of P in H4P2O5,H4P2O6,H4P2O7 respectively are
View Solution