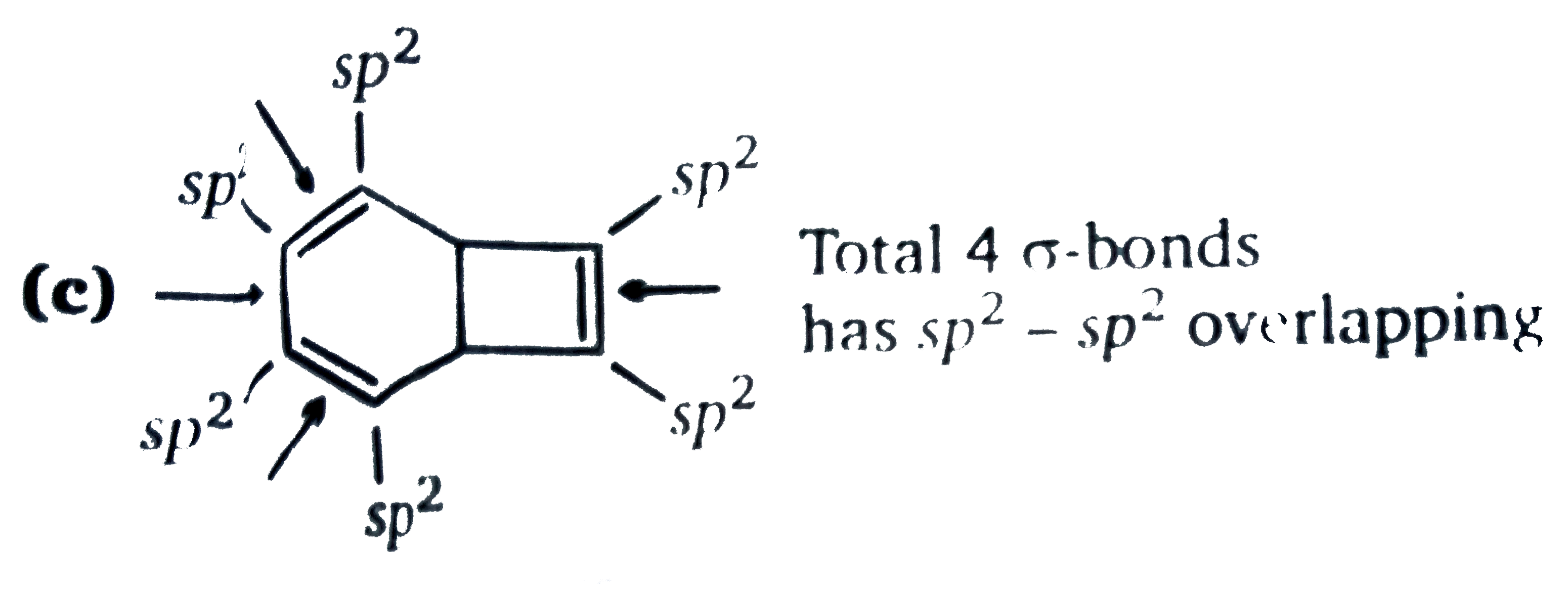The number of sp2−sp2 sigma bonds in the compound given below is :
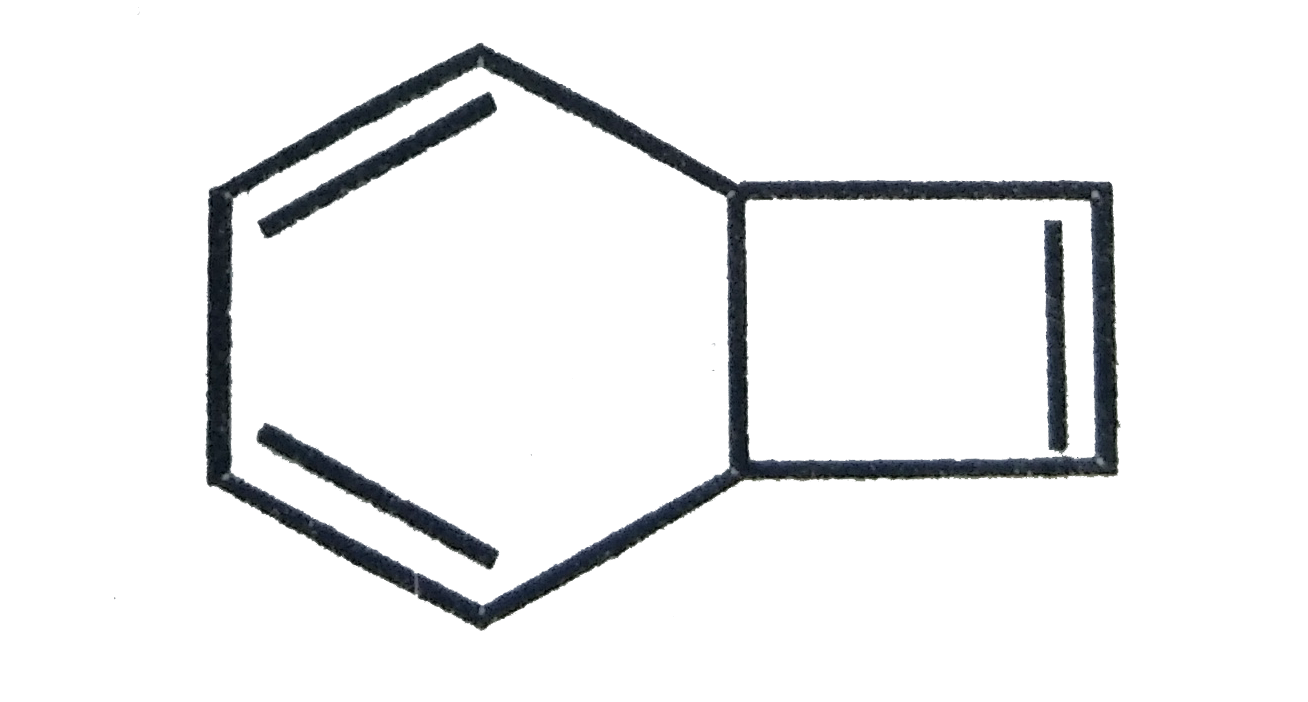
A
1
B
3
C
4
D
5
Video Solution
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Number of sp2−sp sigma bonds in the given compound is
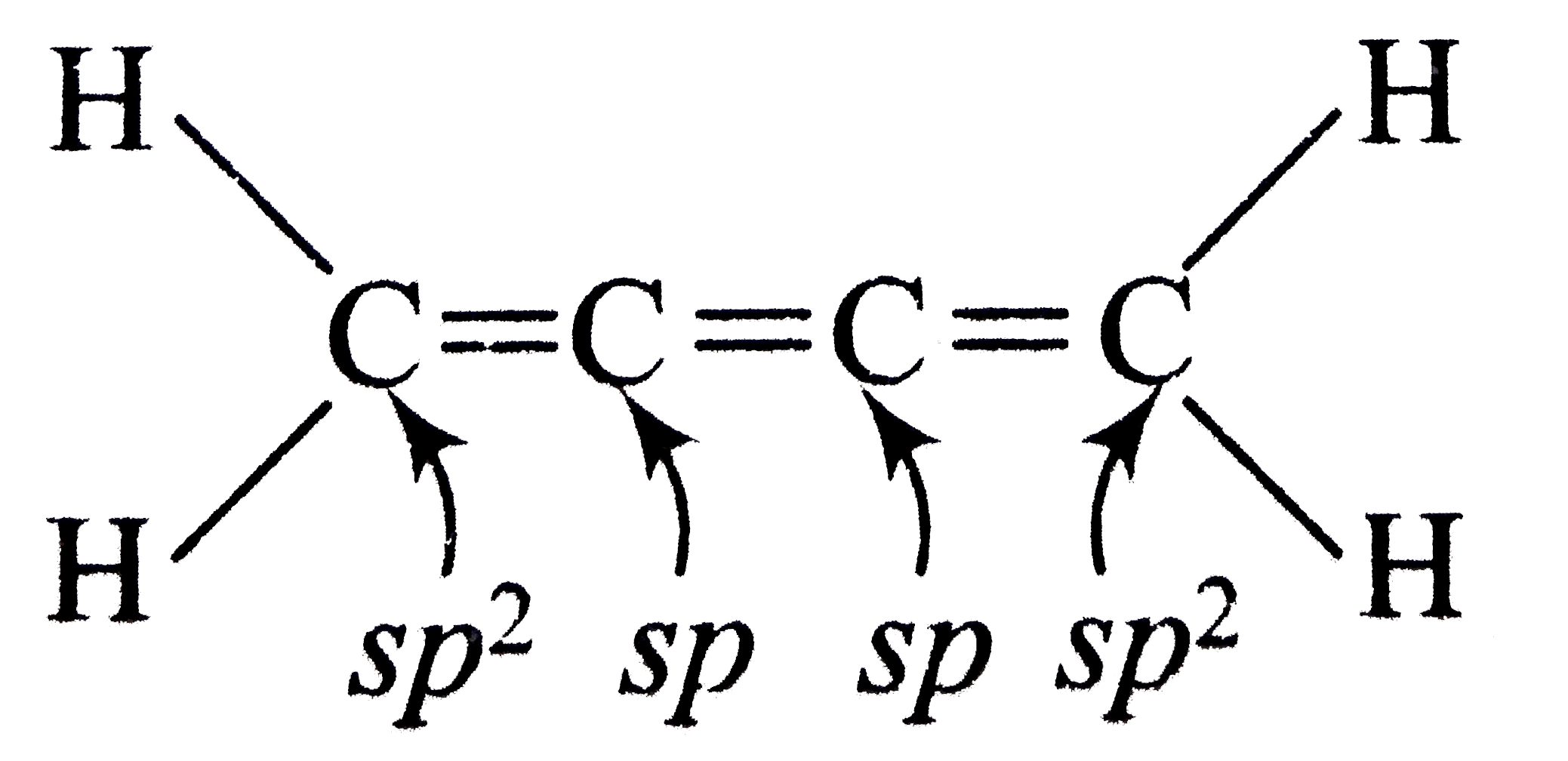 A1B2C3D4
A1B2C3D4The number of sp2−s sigma bonds in benzene are
A3B6C12DnoneThe number of sp2−s sigma bonds in benzene are
A3B6C12Dnone
The ratio of sp3,sp2 and sp carbons in the compound CH3−CH=CH−C≡C−CH3 is
View SolutionThe number of sp2−s sigma bonds in benzene are
View SolutionThe ratio of the number of sp, sp2, and sp3 orbitals in the compound is
CH3−CH=C=CH−C≡C−CH3
View SolutionThe number of sp2 and sp3 hybridized carbon atoms in fructose are respectively.
View SolutionThe outer orbitals of C in ethene molecule can be considered to be hybridized to given three equivalent sp2 orbitals. The total number of sigma (σ) and pi (π) bonds in ethene molecule is
View Solution