Arrange the following in increasing order of their acidic strength.
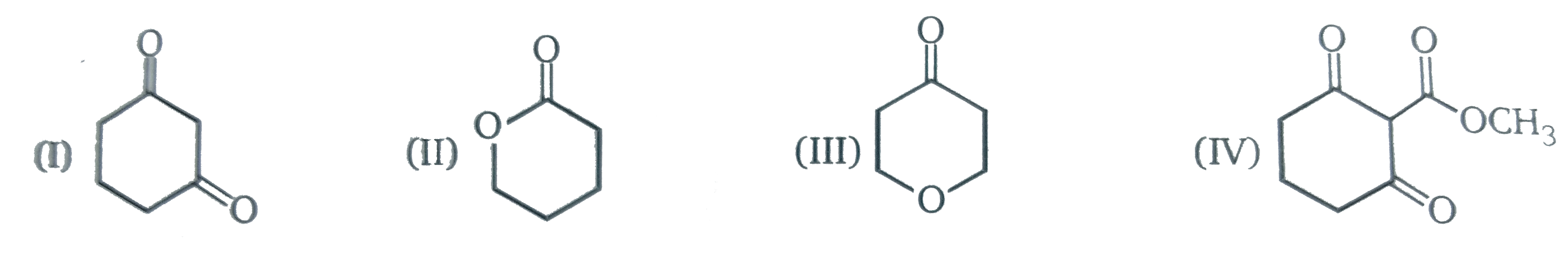
A
III<I<IV<II
B
II<I<IV<III
C
I<III<IV<II
D
II<III<I<IV
Video Solution
Text Solution
The correct Answer is:D
Acidic stength ∝ stability of resulting anion.
|
Similar Questions
Knowledge Check
Arrange the following in decreasing order of their acidic strengths .
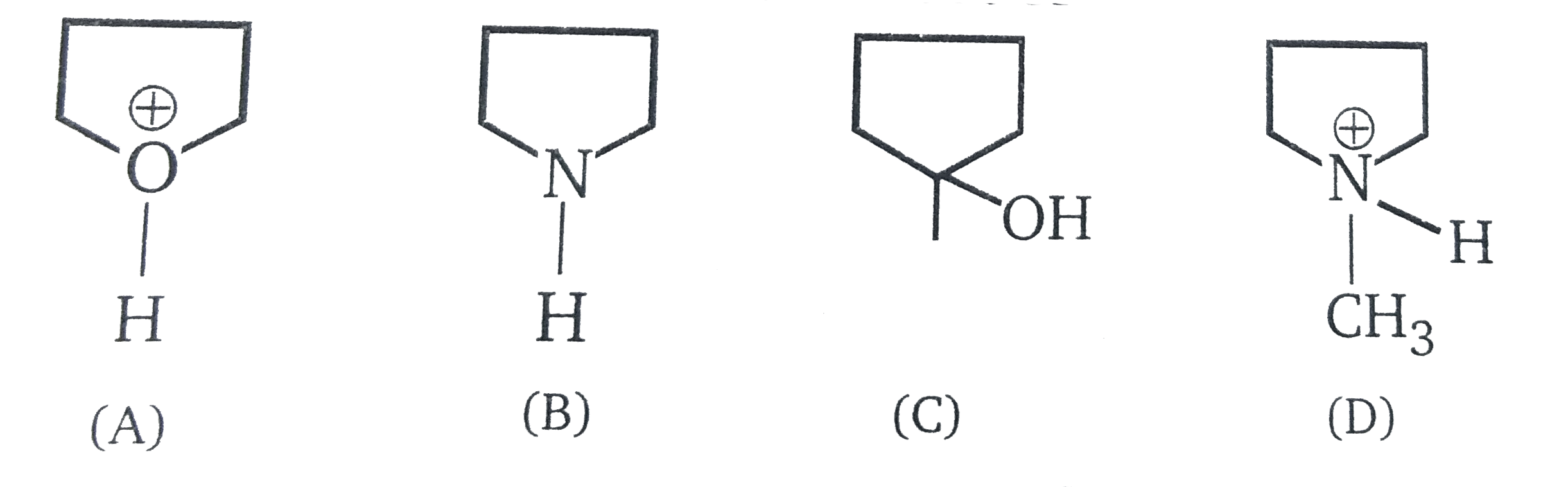 AA>C>B>DBA>D>B>CCA>D>C>BDD>A>C>B
AA>C>B>DBA>D>B>CCA>D>C>BDD>A>C>BArrange the following in the decreasing order of their acidic strength
HClO, HBrO, HIOAHBrO>HClO>HIOBHClO>HBrO>HIOCHIO>HBrO>HClODHClO>HIO>HBrOArrange the following in increasing order of acidic strength
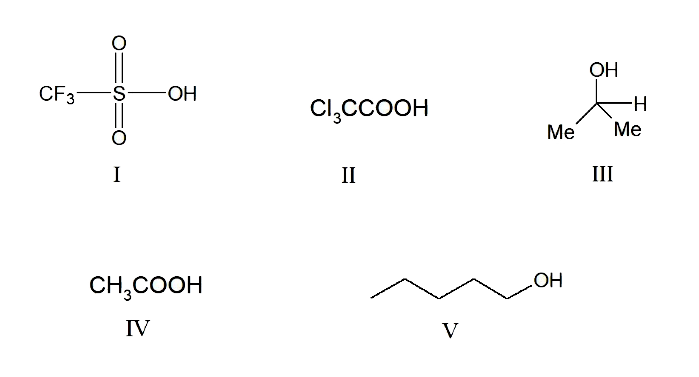 AIII < V < IV < II < IBV < IV < III < II < ICIII < II < IV < I < VDI < II < III < IV < V
AIII < V < IV < II < IBV < IV < III < II < ICIII < II < IV < I < VDI < II < III < IV < V
Arrange the following in the increasing order of acidic strength.
Acetic acid, water and hydrochloric acid.
View SolutionArrange the following in increasing order of acidic strength and explain ltbr? BCl3,BF3,BBr3.
View SolutionArrange the following in the increasing order of acidic strength
(i) FSO3H (ii) HCO−3 (iii) H2O (iv) HSO−4
View SolutionArrange the following in increasing order of acidic strength :
(CH3)2CHCOOH(I),CH3CH2CH(Br)COOH(II),CH3CH(Br)CH2COOH(III)
View SolutionArrange the following in the increasing order of acidic strength :
(i) ClCH2COOH
(ii) CH2ClCH2COOH
(iii ) FCH2COOH
(iv) CH3COOH
View Solution
