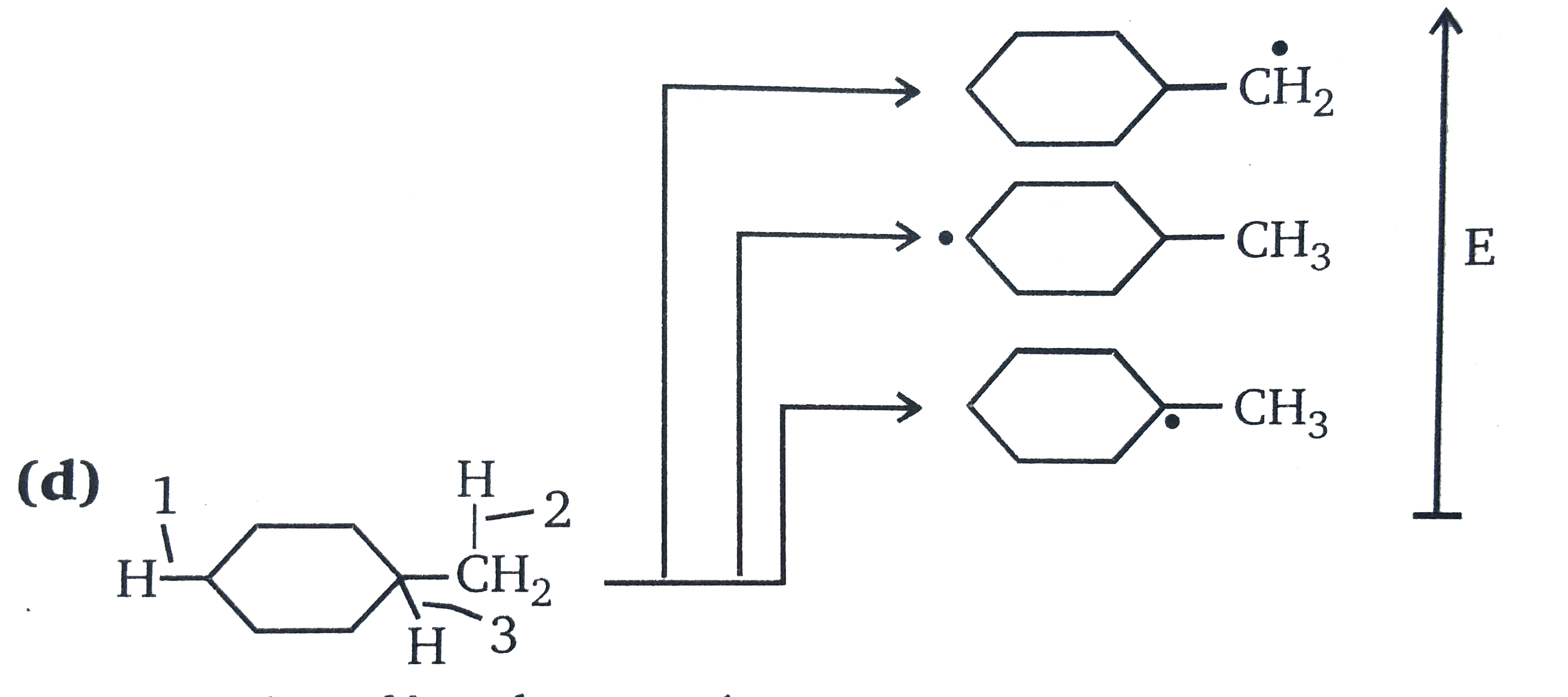
Rank the bond dissociation energies of the bond indicated with the arrows. (form smallest to largest )
A
1<2<3
B
3<2<1
C
2<3<1
D
3<1<2
Video Solution
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
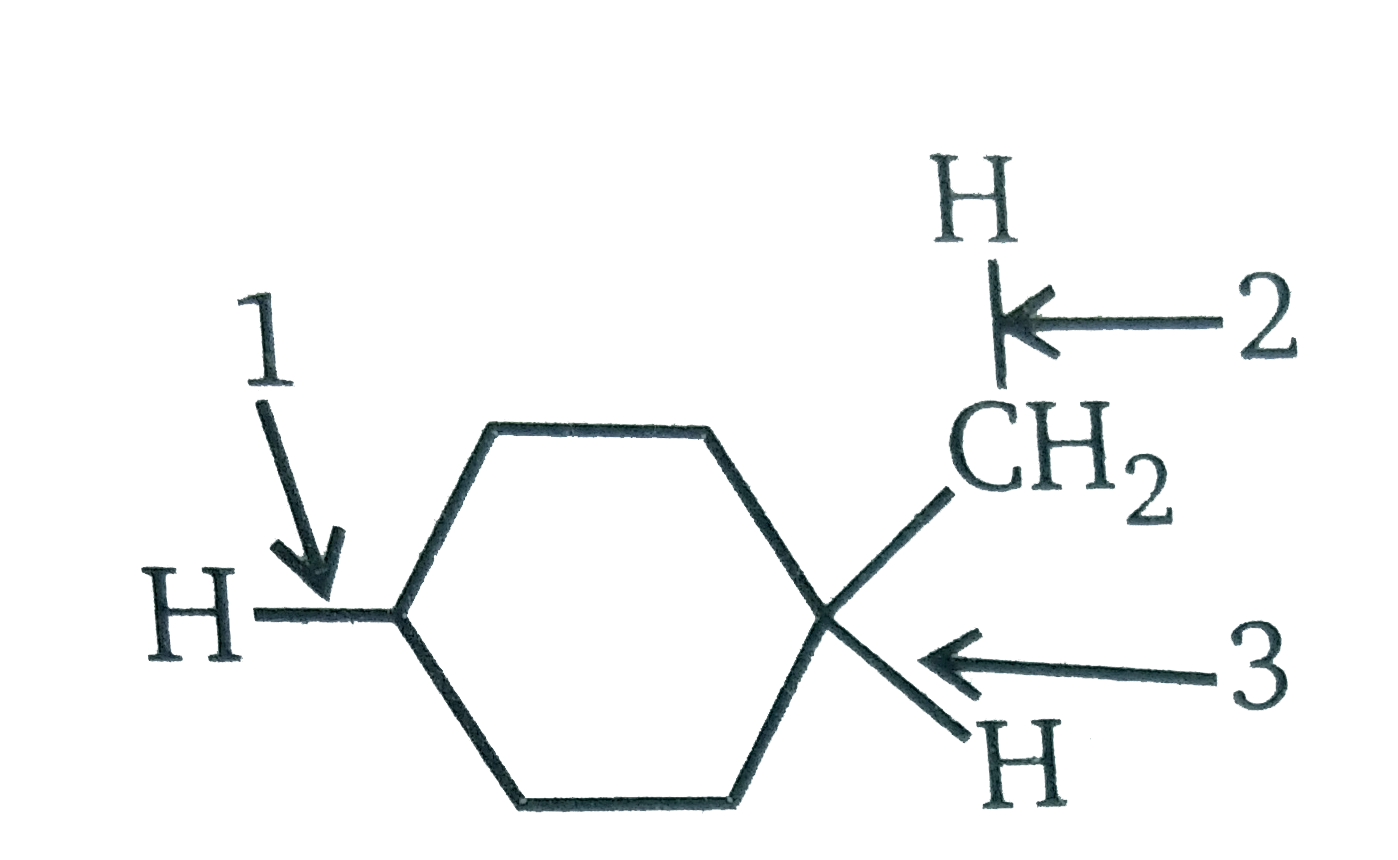
Rank the bond dissociation energies of the bond indicated with the arrows. (form smallest to largest )
 A1<2<3B3<2<1C2<3<1D3<1<2
A1<2<3B3<2<1C2<3<1D3<1<2Rank the bond dissociation energies of the bonds indicated with the arrows, (from smallest to largest)
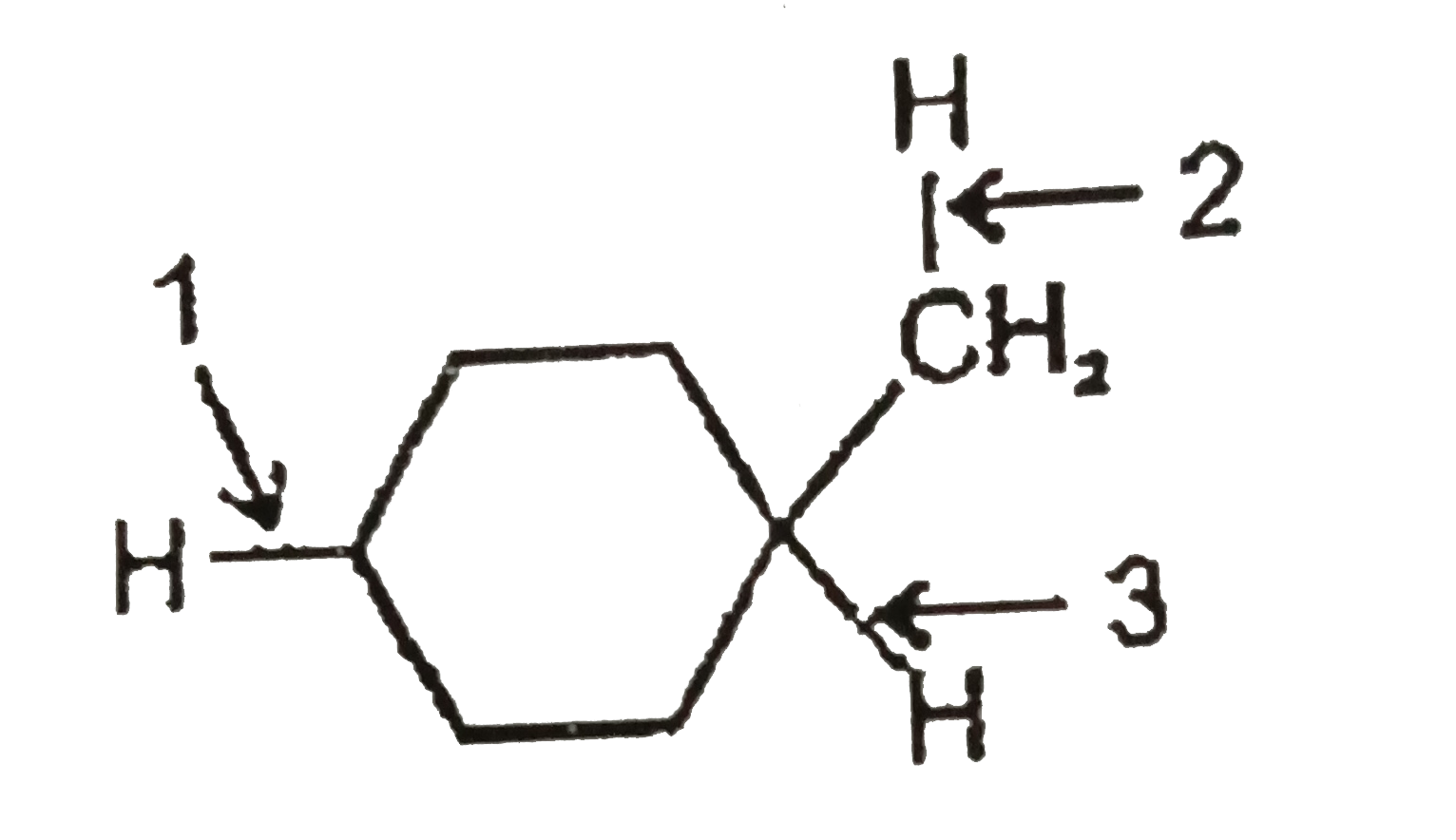 A1<2<3B3<2<1C2<3<1D3<1<2
A1<2<3B3<2<1C2<3<1D3<1<2Halogen with lowest bond dissociation energy is
AF2BBr2CCl2DAll have nearly equal bond energies.
The X - X bond dissociation energy is minimum in:
View SolutionThe bond dissociation energy off2 is very low due to
View SolutionHomolytic bond dissociation energy of C−H bond is minimum for
View SolutionThe bond dissociation energy of the C-H bond for the compound
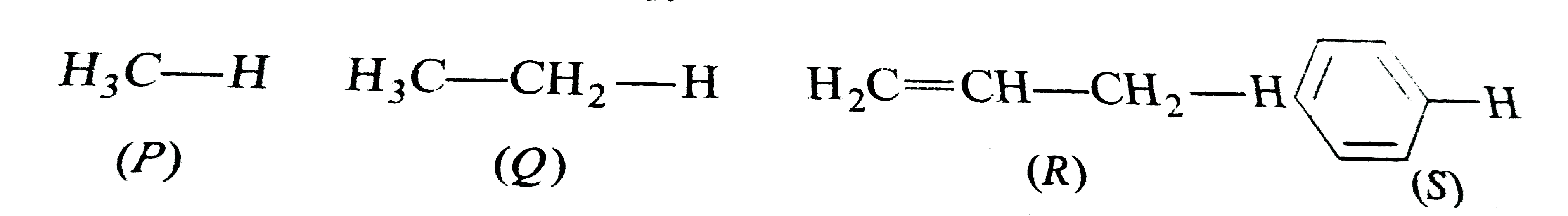
decreases in the order:
View SolutionBond dissociation energies of HF, HCI, HBr follow the order
View Solution