Calculate enantiomeric axcees of mixture containing 6g of (+) 2-butanol and 4g of (-) 2-butanol.
A
0.1
B
0.2
C
0.4
D
0.33
Video Solution
Text Solution
The correct Answer is:b
Optical purity or enantiomeric excess =Exess of one enantiomerTotal mixture×100
Enantiomeric exess (e.e.) =6g−4g6+4×100=20%
|
Similar Questions
Knowledge Check
The enantiomeric excess and observed rotation of a mixture containing 6 gm of (+)-2-butanol and 4 gm of (-)-2-butanol are respectively (if the specific rotation of enantiomerically pure (+)-2-butanol is +13.5 unit).
A80%, + 2.7 unitB20%, -27 unitC20%, + 2.7 unitD80%, - 27 unitDehydration of 2-butanol yield
A1-buteneB2-buteneC2-butyneDBoth (a) and (b)Which of the following would give (−)−2−butanol ?
A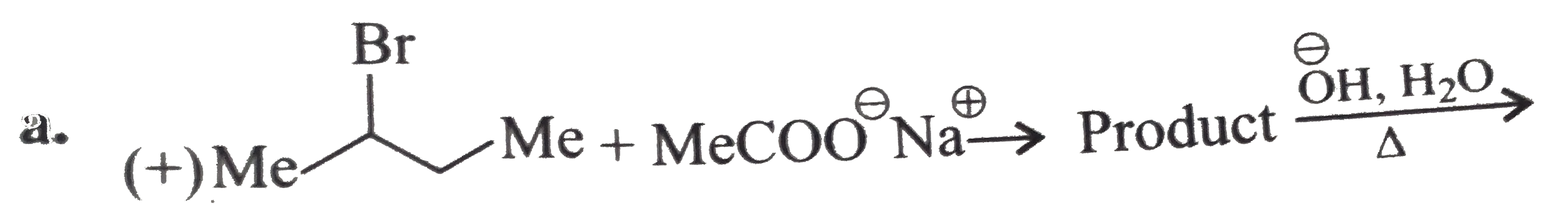 B
B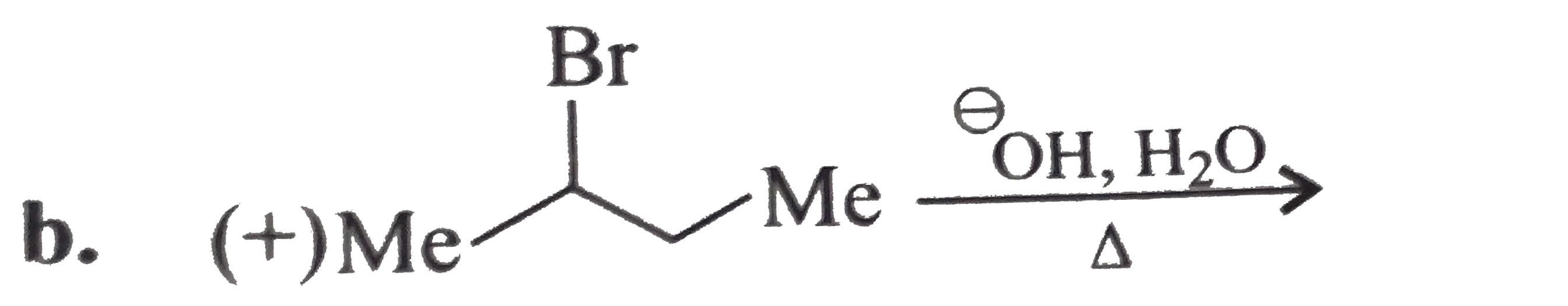 C
C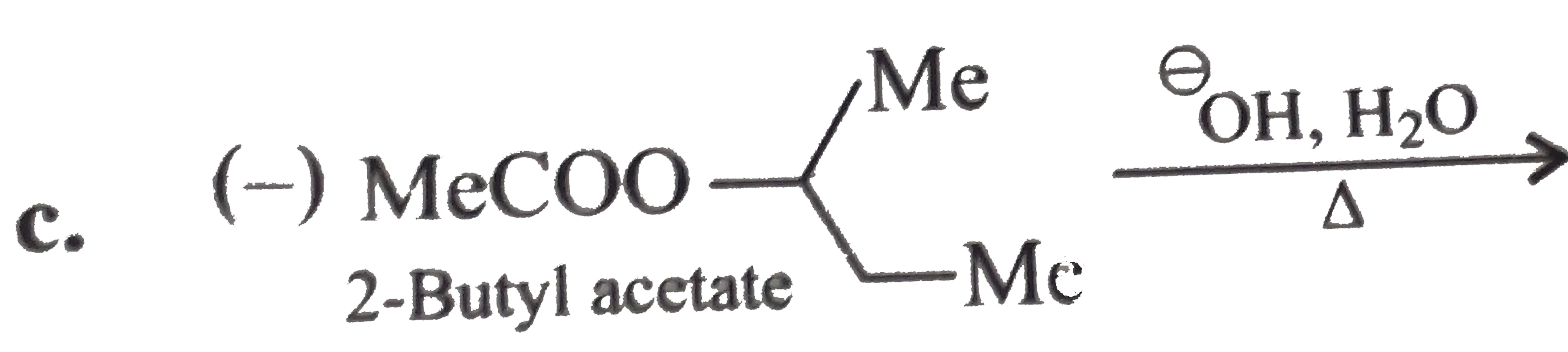 DAll
DAll
3-methyl-2-butanol
View Solution3-methyl-2-butanol
View SolutionThe simplest alcohol that can exhibit enantiomerism is
(A) "1" -propanol
(B) "2" -butanol
(C) "2" -propanal
(D) "1" -butanol
View SolutionAcetaldehyde to butan-1-ol
View SolutionCalculate the partial pressures of each gas present in a mixture of 8 g of oxygen and 6 g of hydrogen, present in 2 L container at 27∘C .
View Solution
