Which of the following compound is achiral ?
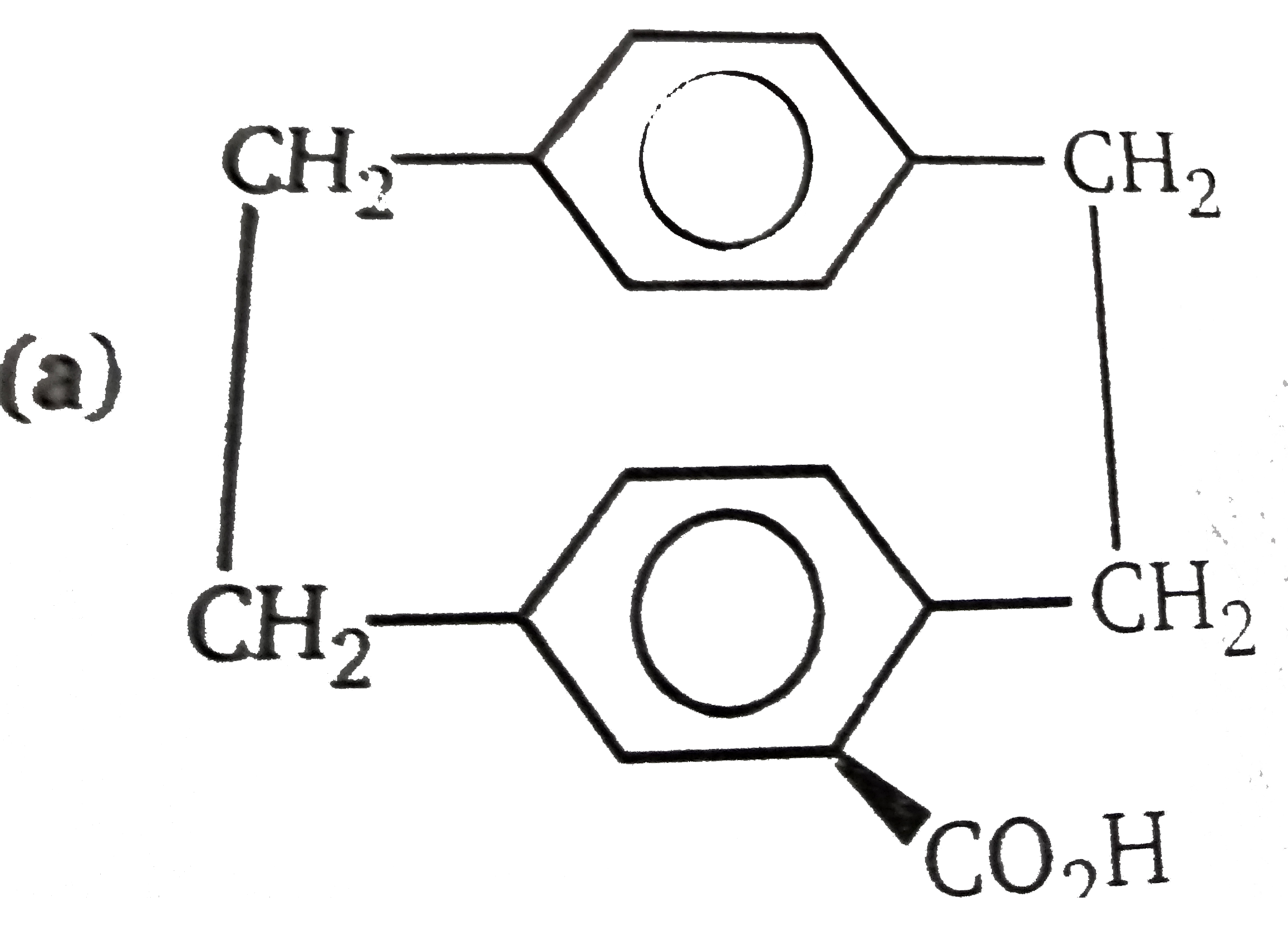
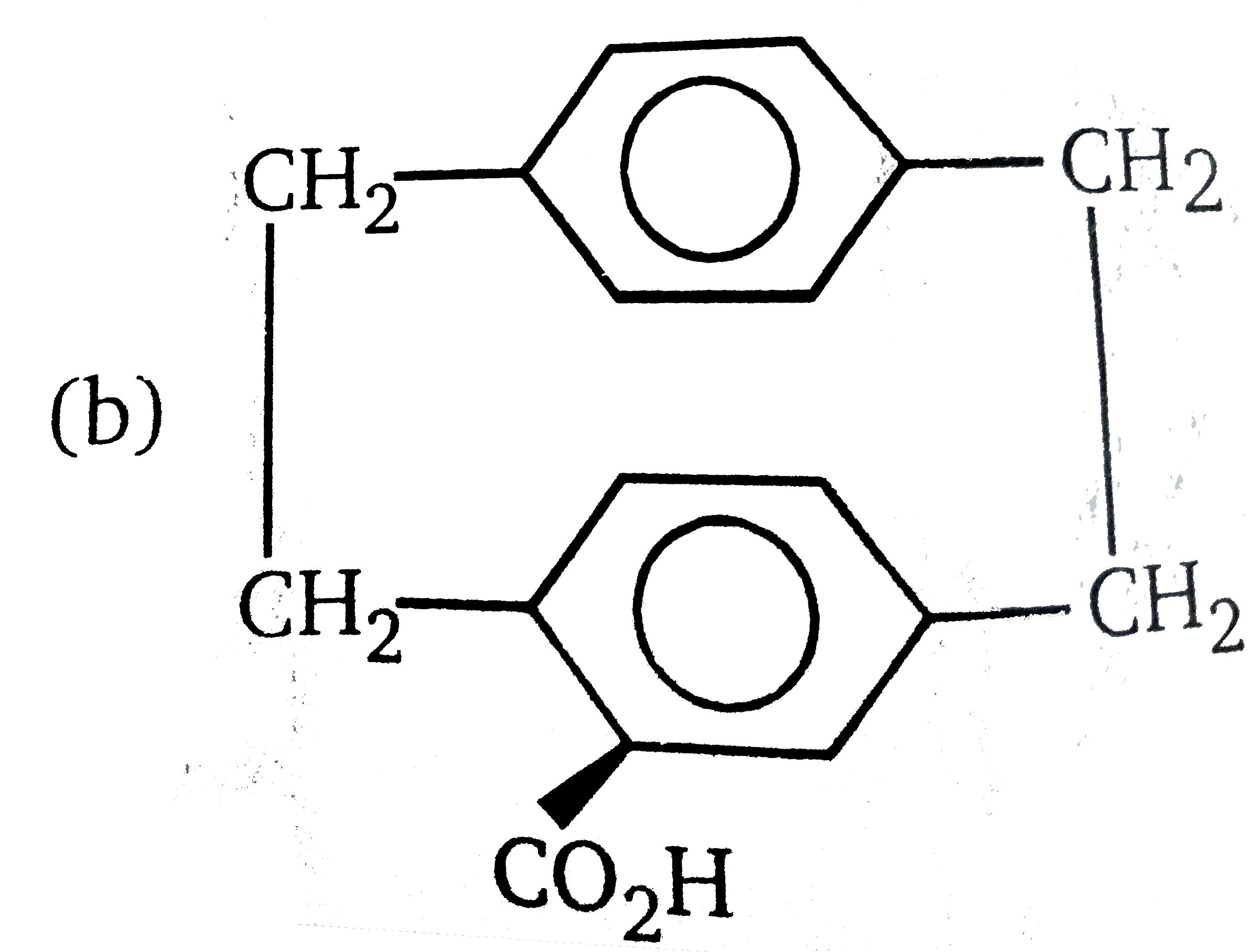
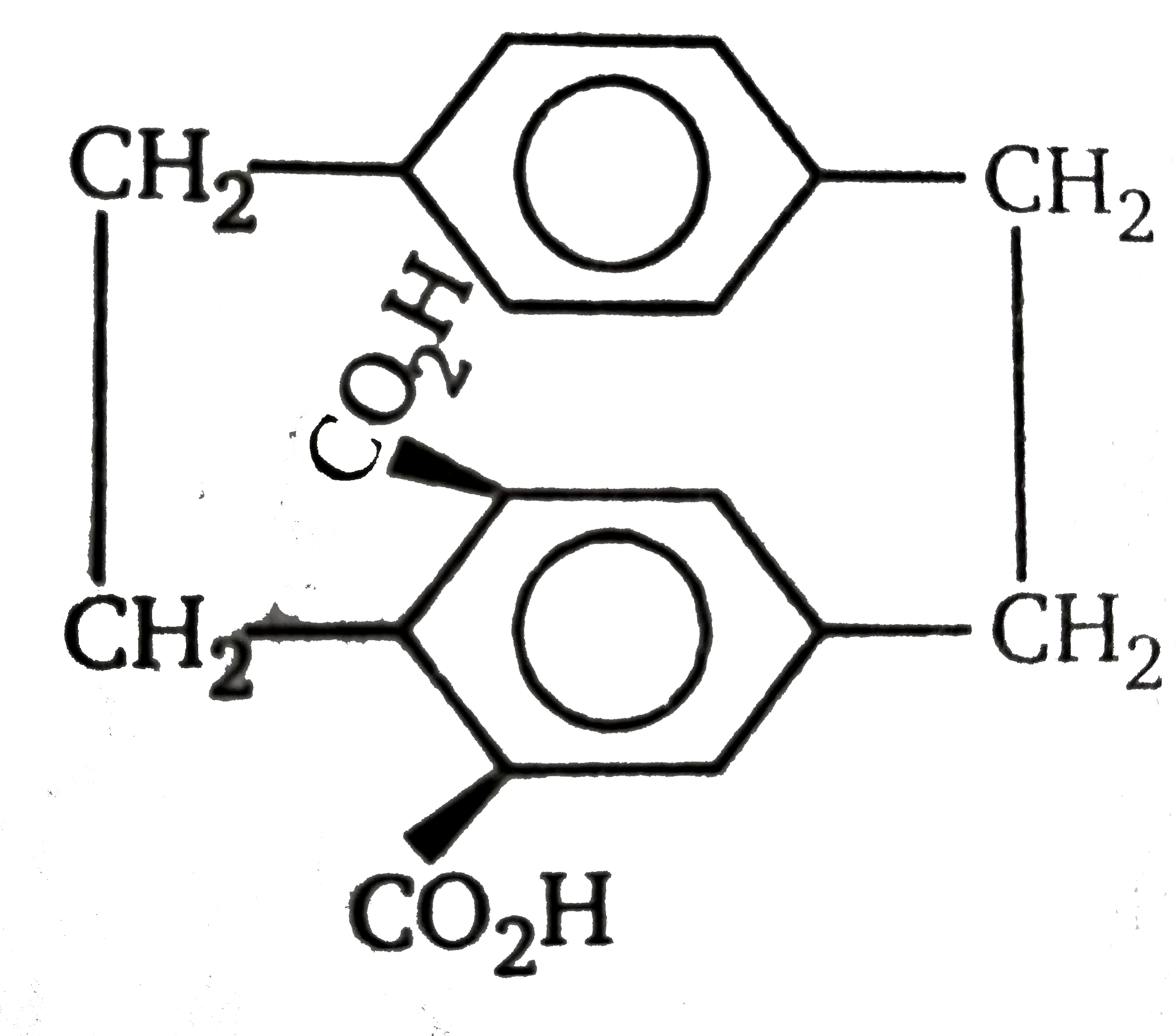

The correct Answer is:c
To determine which of the given compounds is achiral, we need to understand the concept of chirality and the presence of symmetry in molecular structures.
1. Understanding Chirality:
- Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image. A chiral molecule lacks a plane of symmetry.
- Achiral compounds, on the other hand, are optically inactive and possess at least one plane of symmetry, making them superimposable on their mirror images.
2. Identifying Symmetry Elements:
- To determine if a compound is achiral, we need to look for symmetry elements such as a plane of symmetry, center of symmetry, or axis of symmetry.
- If a compound has a plane of symmetry, it is likely to be achiral.
3. Analyzing the Compounds:
- Examine each compound provided in the question. For each compound, check if there is a plane of symmetry.
- For example, if a compound has identical groups on both sides of a central atom, it may have a plane of symmetry.
4. Conclusion:
- After analyzing the compounds, identify the one that has a plane of symmetry. This compound will be the achiral one.
Example Analysis:
- Compound A: If it has four different substituents on a carbon atom, it is chiral.
- Compound B: If it has two identical groups on one side and two different groups on the other, it may have a plane of symmetry and could be achiral.
- Compound C: If it has all identical groups, it is achiral.
- Compound D: If it lacks symmetry and has four different substituents, it is chiral.
Final Answer:
Based on the analysis, the compound that has a plane of symmetry is the achiral compound.
---
- Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image. A chiral molecule lacks a plane of symmetry.
- Achiral compounds, on the other hand, are optically inactive and possess at least one plane of symmetry, making them superimposable on their mirror images.
- To determine if a compound is achiral, we need to look for symmetry elements such as a plane of symmetry, center of symmetry, or axis of symmetry.
- If a compound has a plane of symmetry, it is likely to be achiral.
- Examine each compound provided in the question. For each compound, check if there is a plane of symmetry.
- For example, if a compound has identical groups on both sides of a central atom, it may have a plane of symmetry.
- After analyzing the compounds, identify the one that has a plane of symmetry. This compound will be the achiral one.
- Compound A: If it has four different substituents on a carbon atom, it is chiral.
- Compound B: If it has two identical groups on one side and two different groups on the other, it may have a plane of symmetry and could be achiral.
- Compound C: If it has all identical groups, it is achiral.
- Compound D: If it lacks symmetry and has four different substituents, it is chiral.
Based on the analysis, the compound that has a plane of symmetry is the achiral compound.
Similar Questions
Knowledge Check
Which of the following compund is achiral
A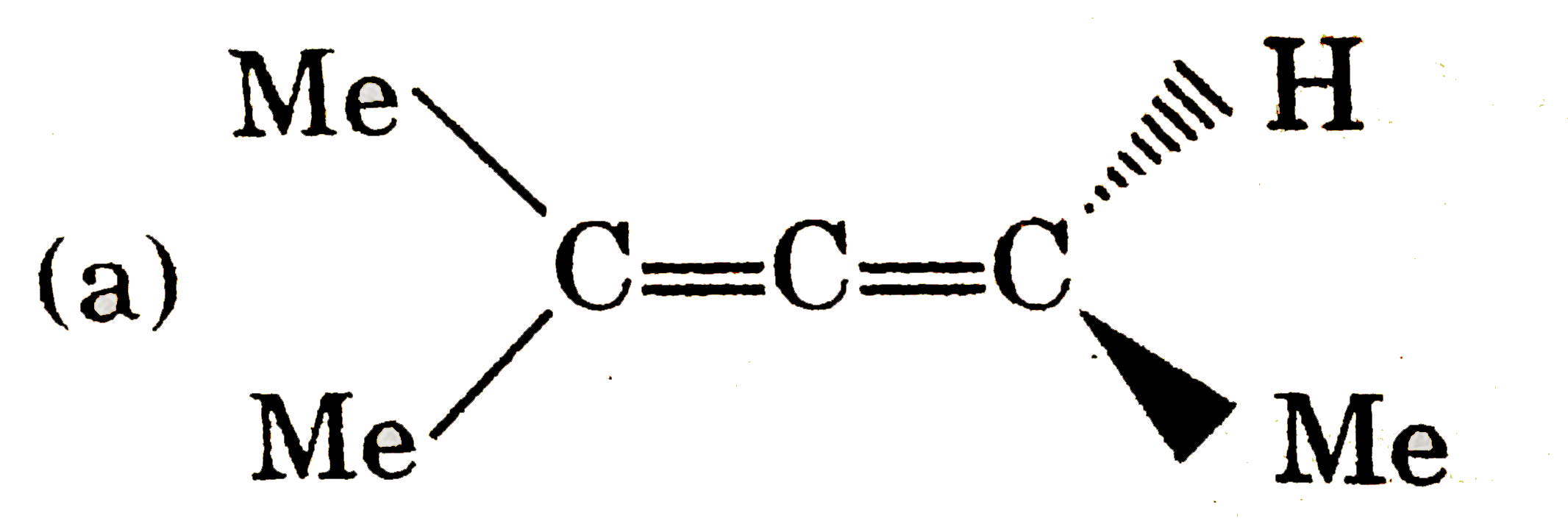 B
B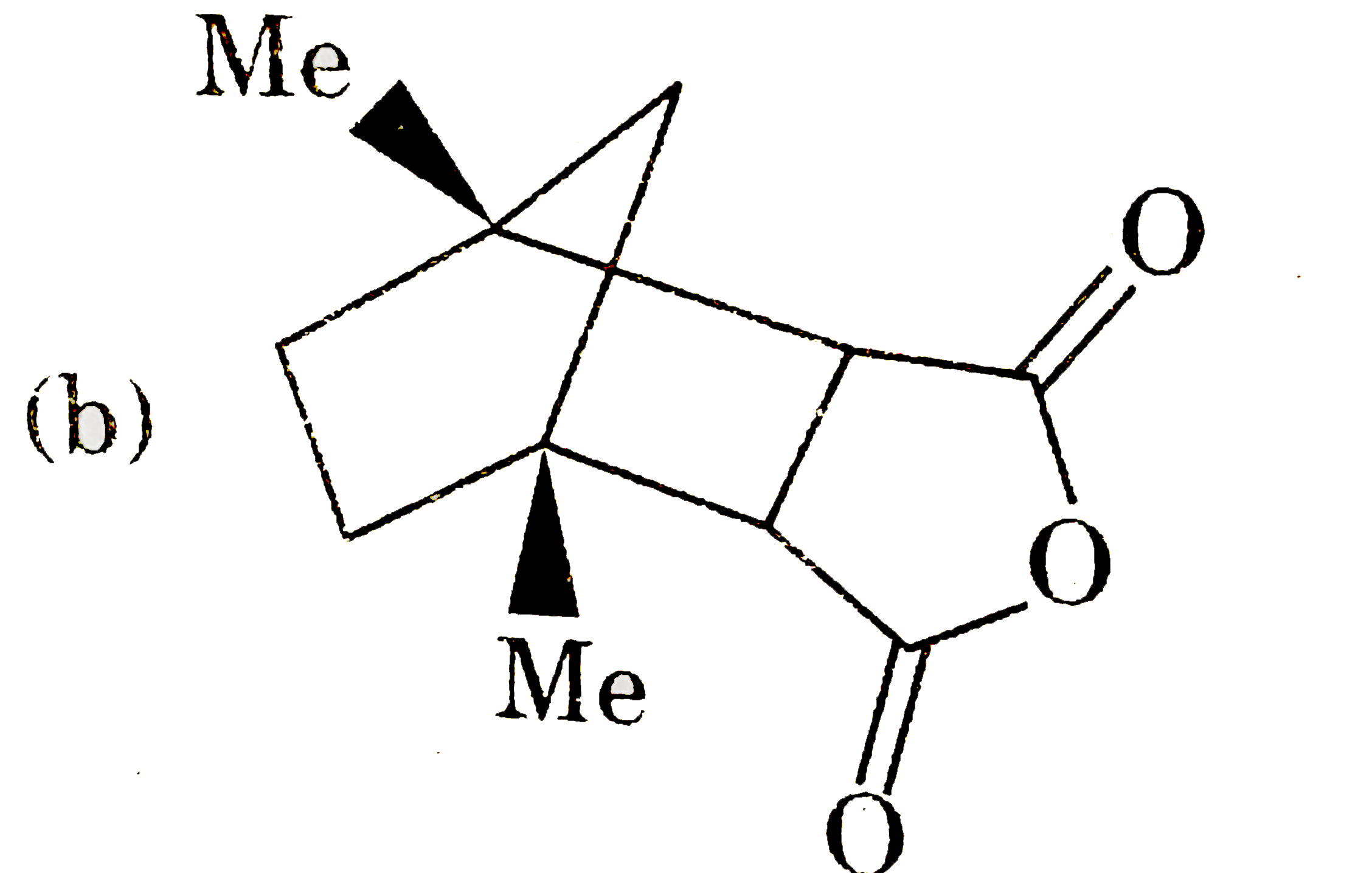 C
C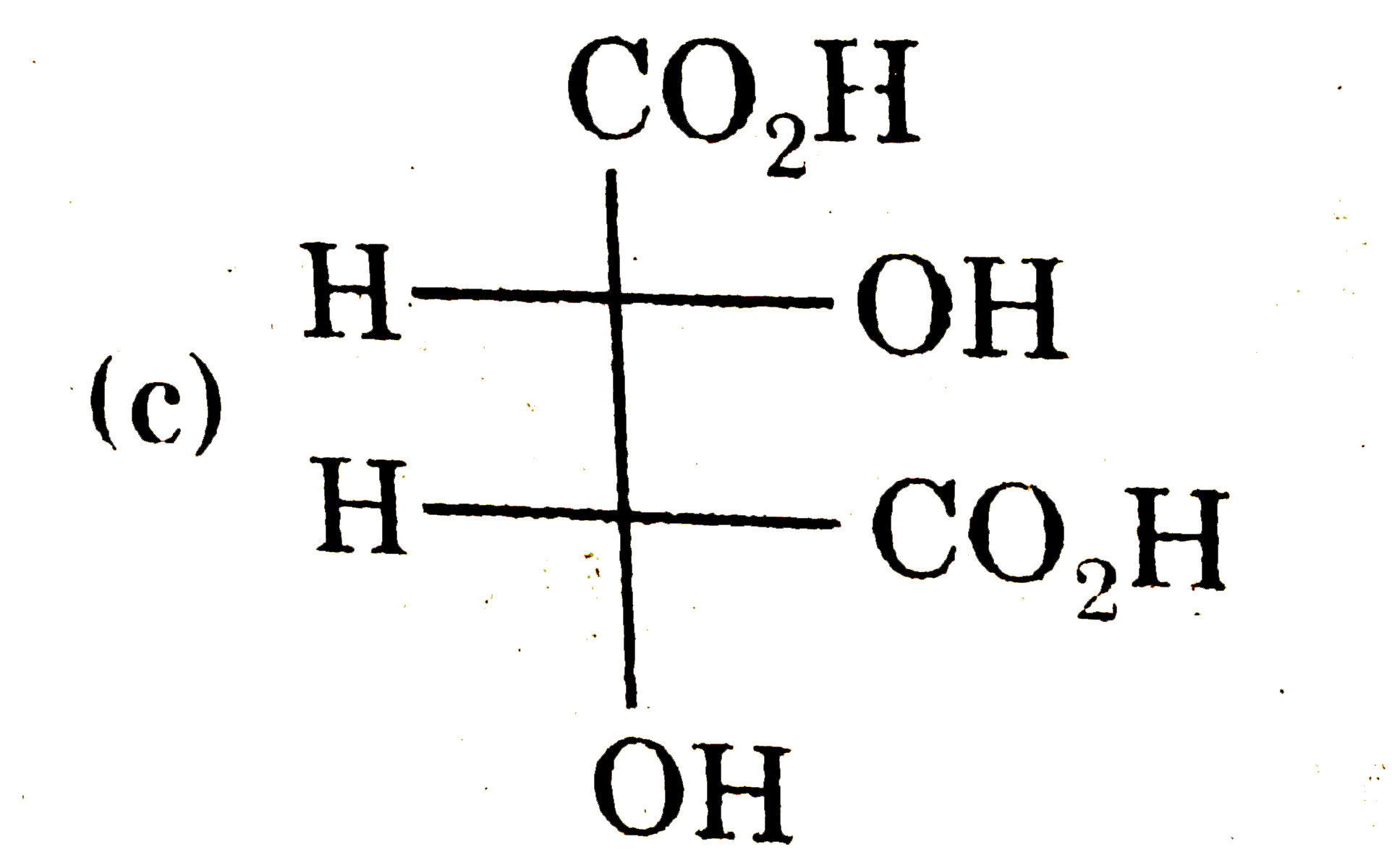 D
D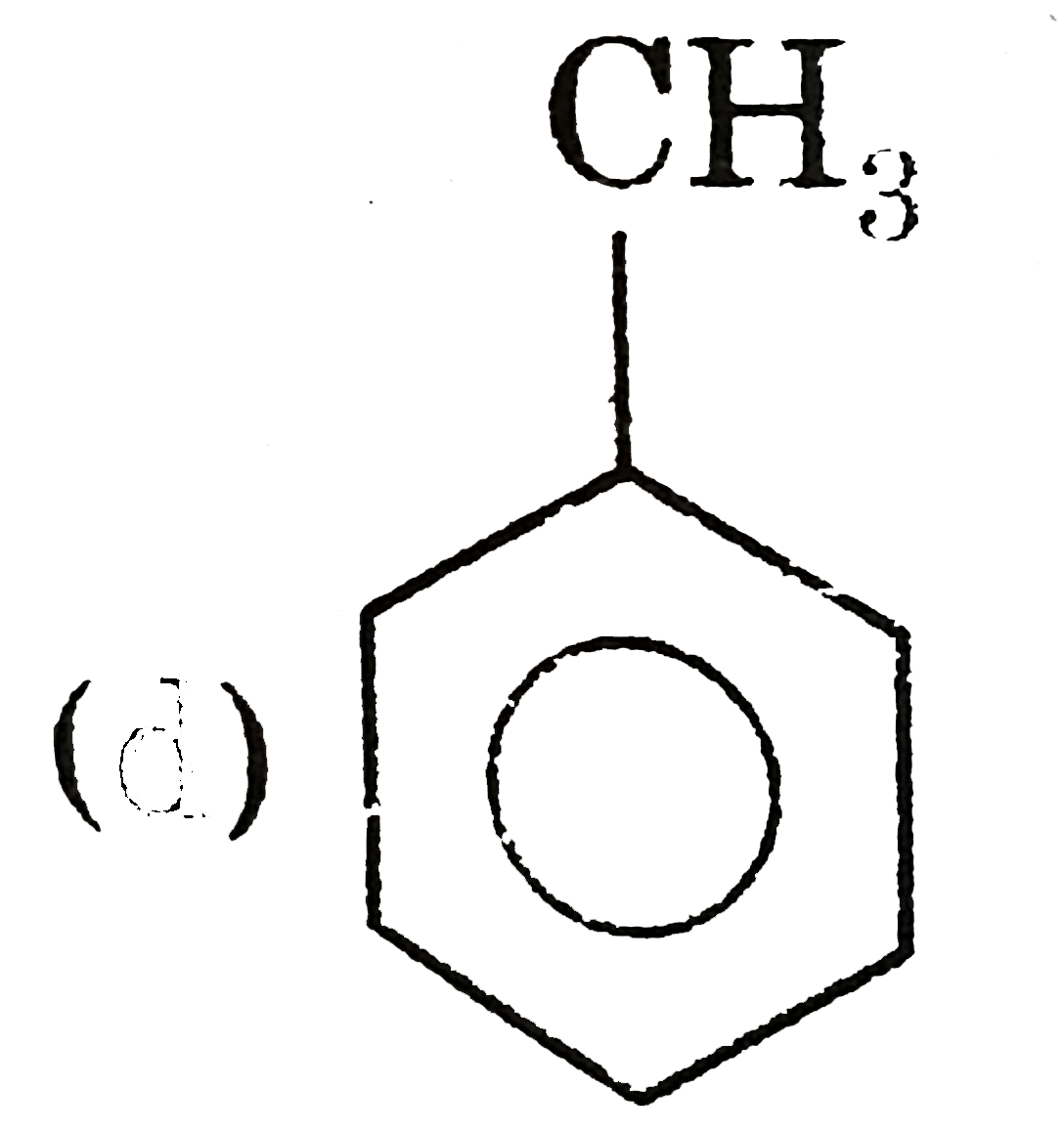
Which of the following compounds is chiral? .
ACH3CH2..NHCH3BCH3CH2CH2CH3∣N..CH2CH2CH3CCH3CH2CH2CH2C6H5∣N+∣CH2CH3Br¯Dall of theseWhich of the following compounds is chiral? .
A3-PentanolB1-PentanolC3-Methyl-1-butanolD3-Methyl-2-butanol
