Aldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box.
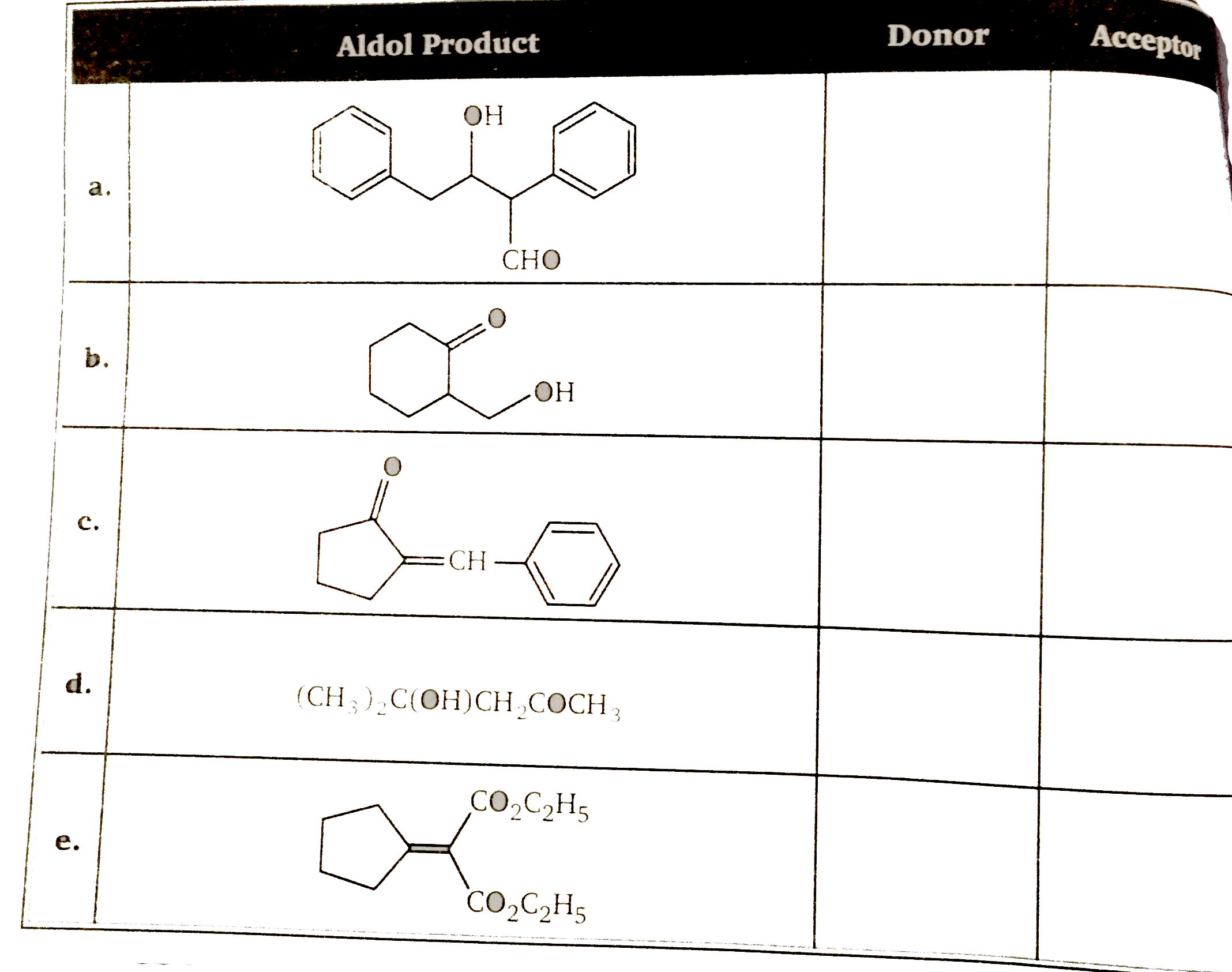 .
.
Q. 
The correct Answer is:a-Donor=C, Acceptor=C;b-Donor=E,Acceptor=D; c-Donor=B,Acceptor=A;d-Donor=G,Acceptor=G;e-Donor=F,Acceptor=B
a-Donar=C,Acceptor=C,b-Donar=E,Acceptor=D,c-Donar=B,Acceptor=A,d-Donar=G,Acceptor=G,e-Donar=F,Acceptor=Bs
Topper's Solved these Questions
- ALDOL AND CANNIZARO REACTIONBOOK - MS CHOUHANCHAPTER - ALDOL AND CANNIZARO REACTIONEXERCISE - Subjective Problems
3 Videos
- ALDOL AND CANNIZARO REACTIONBOOK - MS CHOUHANCHAPTER - ALDOL AND CANNIZARO REACTIONEXERCISE - Level 1 (Q.31 To Q.48)
3 Videos
- ALDEHYDES AND KETONES II. ALDOL REACTIONS BOOK - MS CHOUHANCHAPTER - ALDEHYDES AND KETONES II. ALDOL REACTIONS EXERCISE - ADDITIONAL OBJECTIVE QUESTIONS (LINKED COMPREHENSION TYPE )
6 Videos
- ALKENES AND ALKYNES IIBOOK - MS CHOUHANCHAPTER - ALKENES AND ALKYNES IIEXERCISE - ADDITIONAL OBJECTIVE QUESTIONS (Matrix Match Type)
2 Videos
Similar Questions
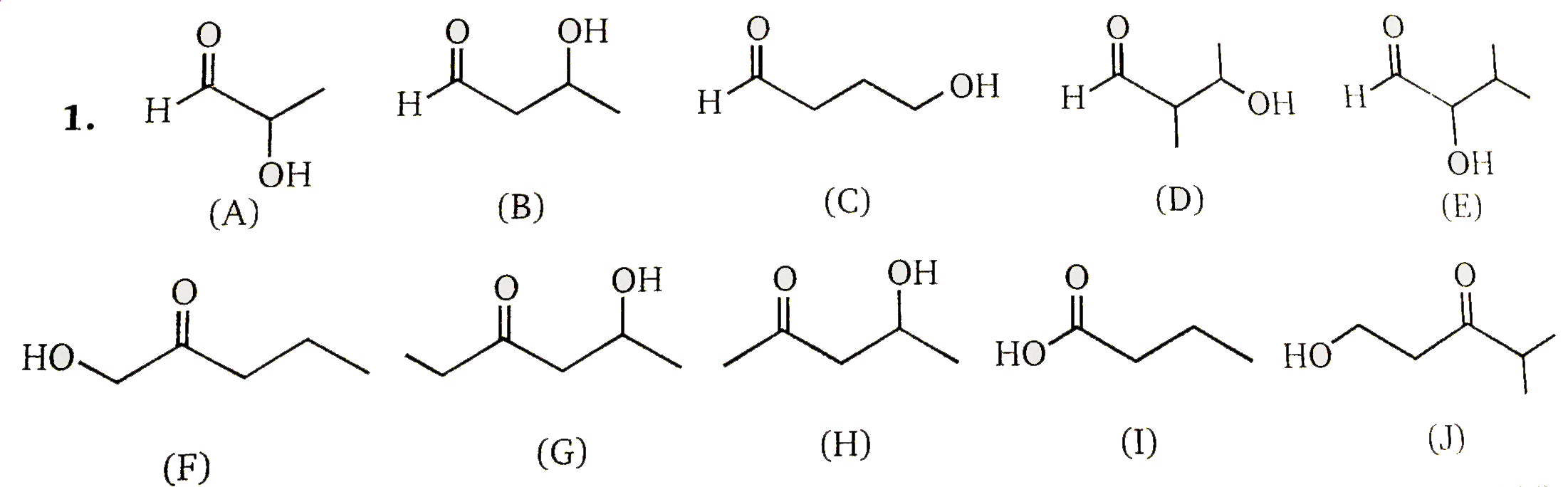
X=number of compound obtained by aldol reaction
Y=Number of compounds react with NaHCO3
Sum of X+Y is
View Solutionx=no of compound better hydride donor than Ph−Oө∣C∣H−Oө
View SolutionA carbonyl compound P,which gives positive iodoform test, undergoes reaction with MemgBr followed by dehydration to give an Olefin Q. Ozonolysis of Q leads to a dicarbonyl compound R, which undergoes intramolecular aldol reaction to give predominantly S.
The structure of the carbonyl compound P is
View SolutionMatch the following

View SolutionThe following questions refer to the twelve compounds given below. You may enter as many as six choices in each answer box.
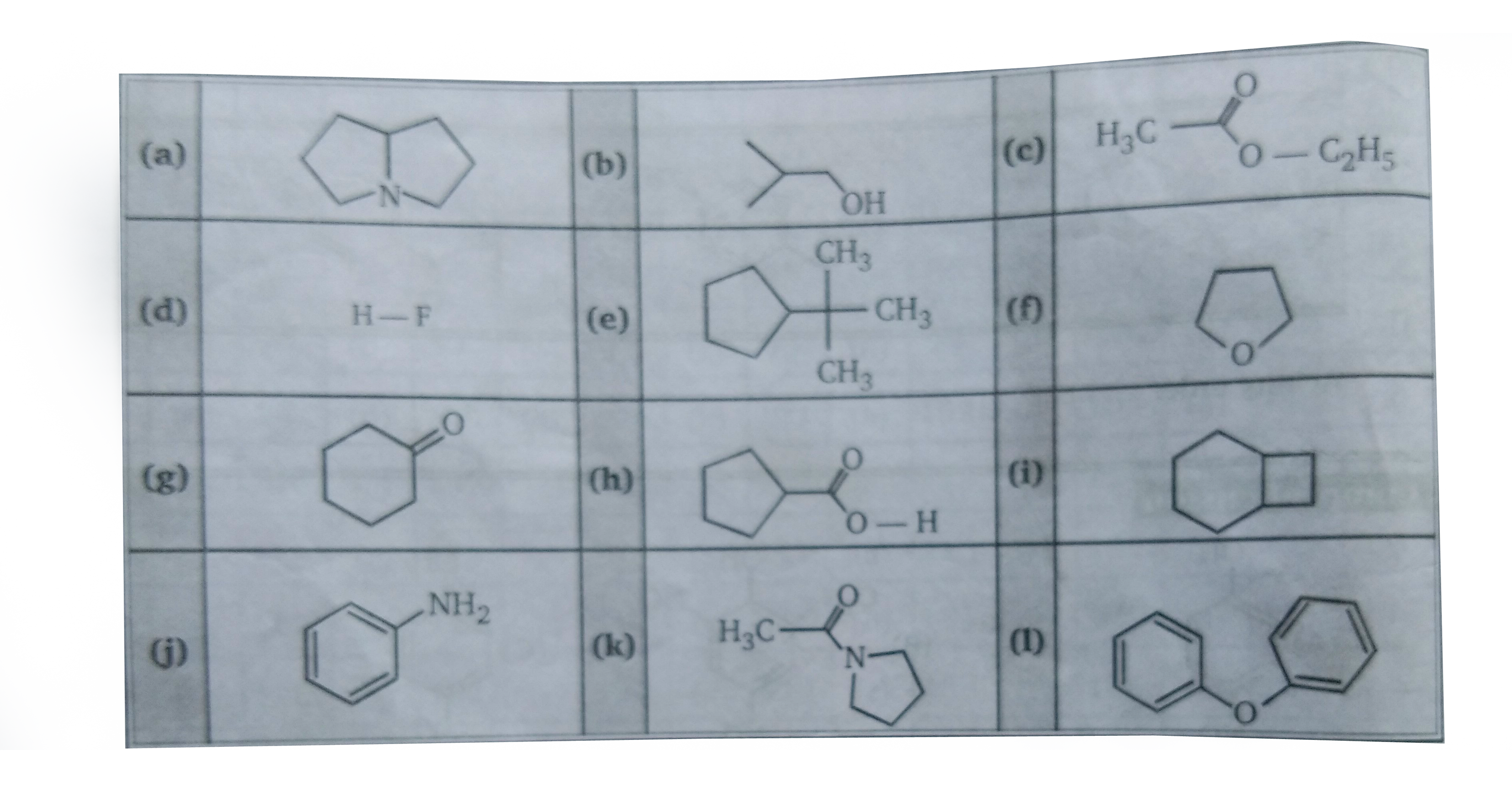
Which may serve both as H-bond donors and acceptors ?
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box.
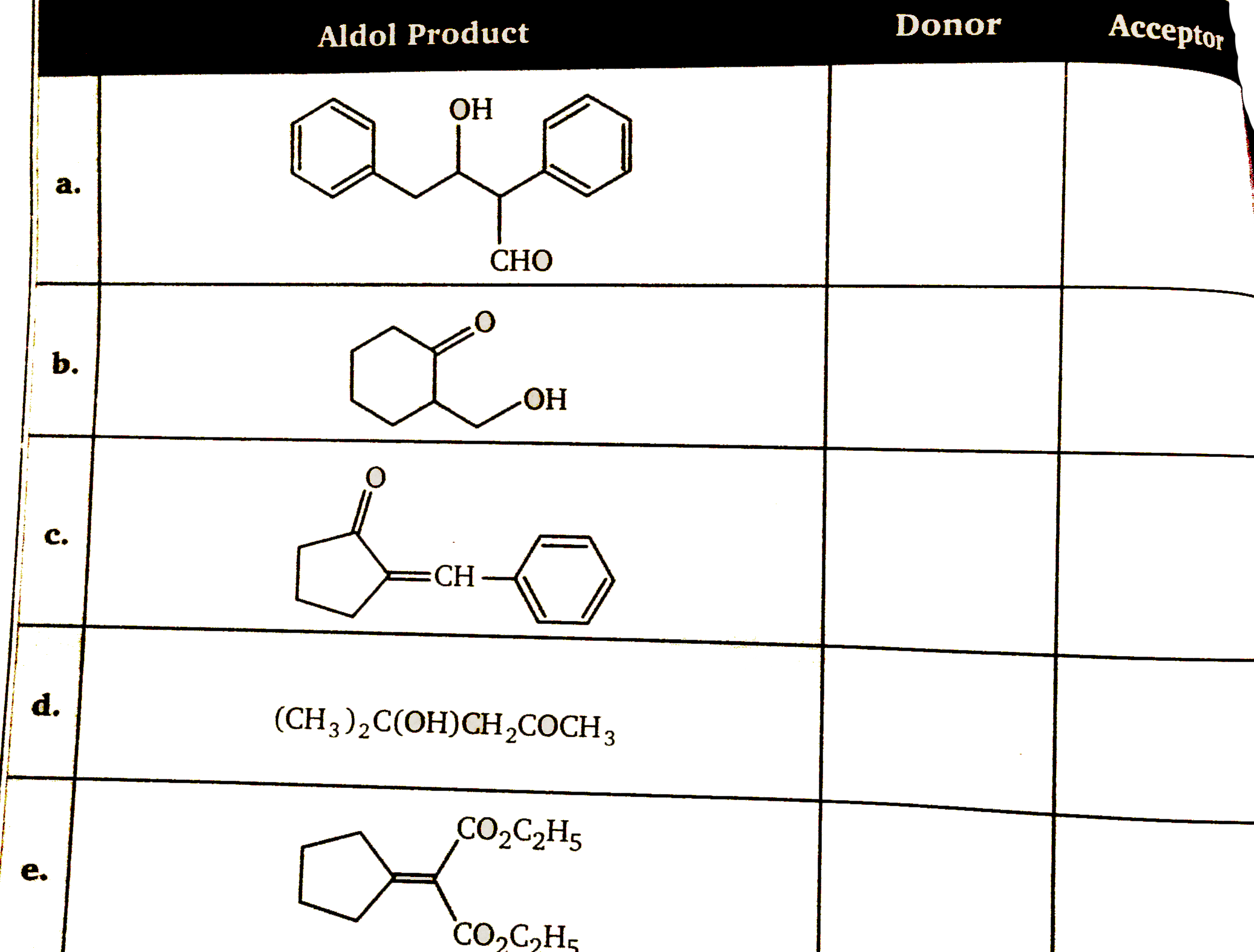 .
.
Q.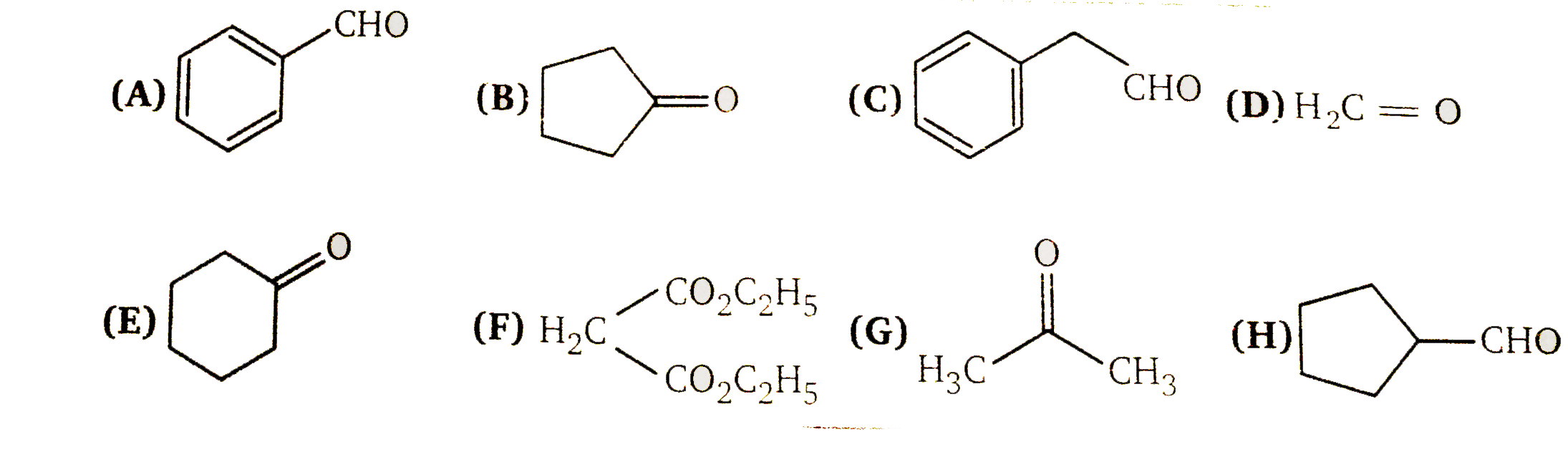
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box. . Q.
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box. .
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box.
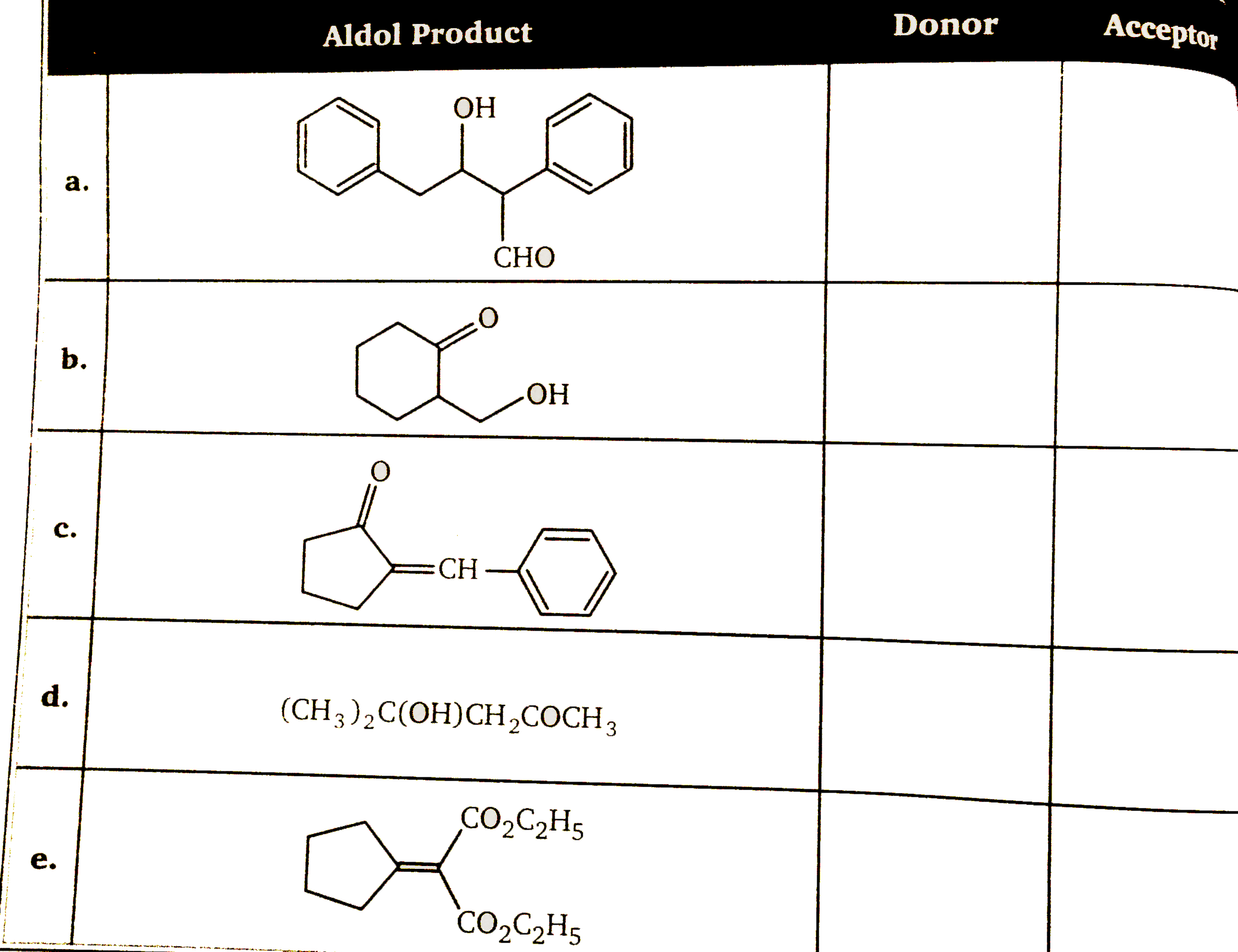 .
.
Q.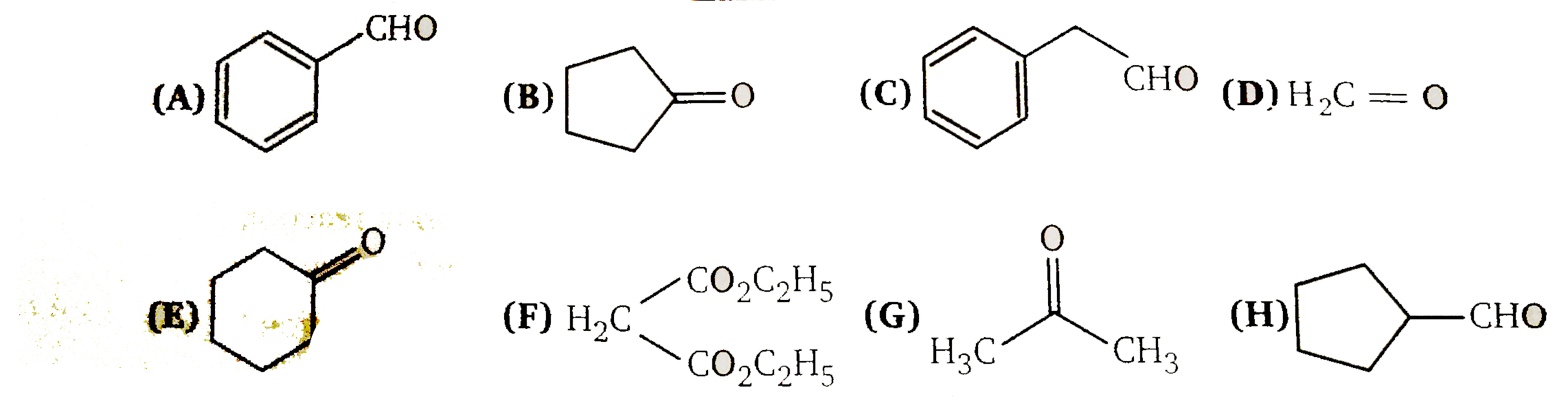
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box.
 .
.
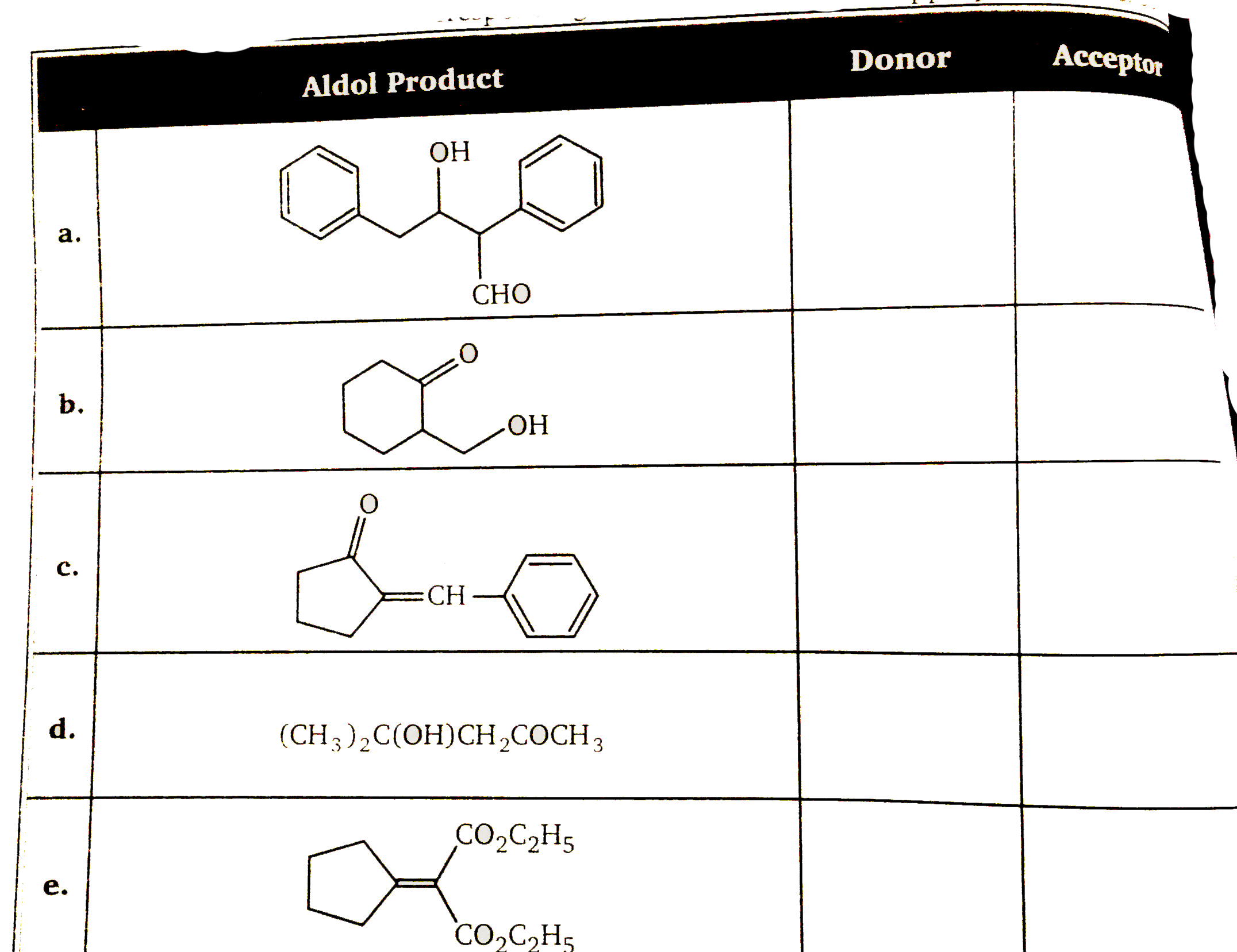
Q.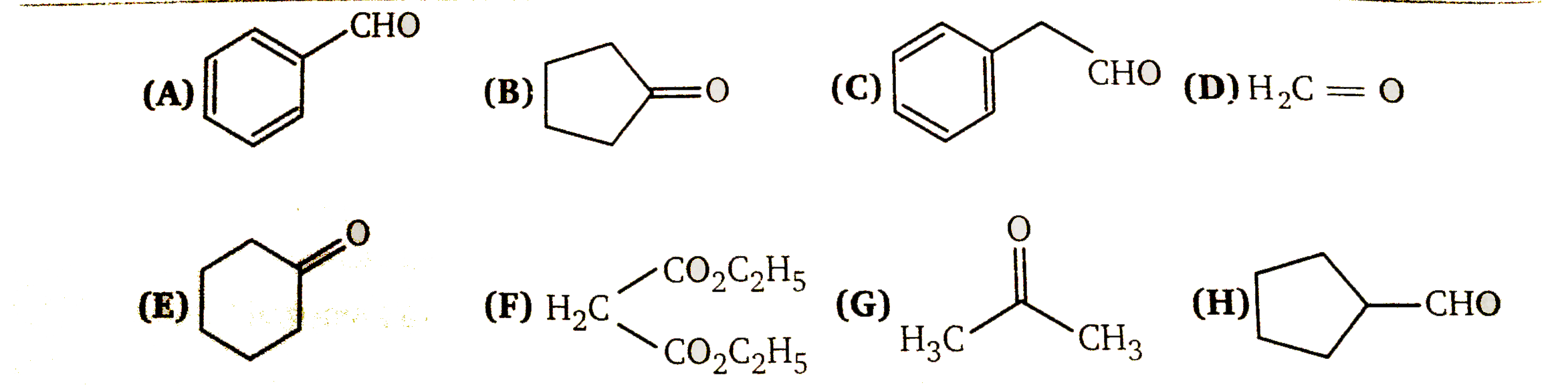
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box. . Q. .
View SolutionAldol condensation proceeds by carbon-carbon bond formation between an enolate donor and a carbonyl acceptor. For each of the following aldol products (a thorugh e) select a donor and an acceptor compound from the list at the bottom of the page (compounds A through H). write the letter corresponding to your selection in the appropriate answer box. . Q.
View SolutionAldol condensation proceeds by carbon- carbon bond formation between an enolation donor and a carbonyl acceptor For each of the following aldol products (1 through 4) Match the donor and acceptor compound.
View SolutionAldol addition is nucleophllic addition reaction of carbonyl compound with enolate or enolate ion of aldehyde and ketones. The reaction between two molecules of acetaldehyde takes place as following in the presence of base.

Driving force for the aldol condensation is :
View SolutionWhich of the following carbonyl compounds undergoes aldol condensation ?
View Solution

