![CH(2)=CHCH(2)-COOH CH(3)CH(2)COOH Arrange the following acids in decreasing order of [H^(+)] conc.](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRB_CHM_ORG_HP_C01_E01_013_Q01.png)
CH2=CHCH2−COOH
CH3CH2COOH Arrange the following acids in decreasing order of [H+] conc.
I>II>III
II>III>I
II>I>III
III>II>I
The correct Answer is:A
Step by step video, text & image solution for CH_(2)=CHCH_(2)-COOH CH_(3)CH_(2)COOH Arrange the following acids in decreasing order of [H^(+)] conc. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Arrange the following compounds in decreasing order of acidity :
CH3CH2OH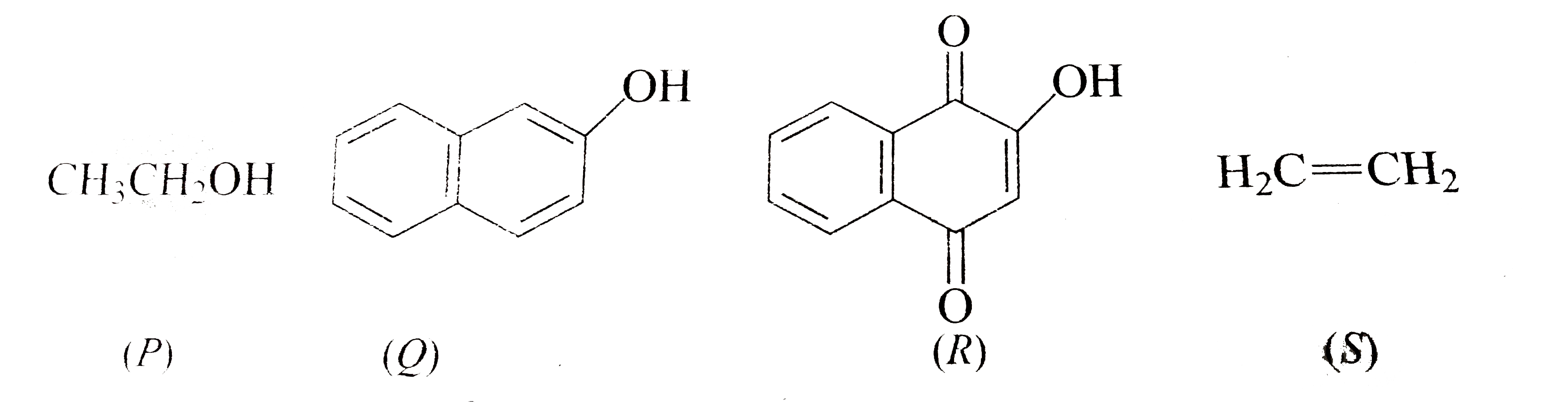
H2C=CH2AR>Q>P>SBQ>R>P>SCS>R>Q>PDQ>P>R>SAmong the aicd
(1) HC≡C−COOH
(2) H2C=CH−COOH
(3) CH3−CH2−COOH
The acidic strength follows the orderA3<2<1B3=2<1C1<2<3D1<2=3(i) F3C−COOH, (ii) CH3COOH, (iii) C6H5COOH, (iv) CH3CH2COOH
Correct order of pKa value is :A1 gt 3 gt 2 gt 4B4 gt 2 gt 3 gt 1C4 gt 3 gt 2 gt 1D1 gt 2 gt 4 gt 3
Why is CH2=CH−COOH a stronger acid than CH3CH2COOH?
View SolutionCH2COOH∣C(OH)COOH∣CH2COOH
View SolutionArrange the following :
(i) −NO2,−COOH,−F,−CN,−I, in decreasing order of -I effect.
(ii) CH−3,D−,(CH3)3C−,(CH3)2CH−,CH3CH2−, in increasing order of +I effect.
View SolutionGive chemical tests to distinguish between
(i) Propanal and propanone
(ii) Benzaldehyde and acetophenone
(b) Arrange the following compounds in an increasing order of their property as indicated :
(i) Acetaldehyde, Acetone , Methyl tert-butyl ketone (reactivity towards HCN)
(ii) Benzoic acid ,3,4-Dinitrobenzoic acid , 4-Metthoxybenzoic acid (acid strength)
(iii) CH3CH2CH(Br)(COOH),CH3CH(Br)CH2COOH,(CH3)2CHCOOH (acid strength)
View SolutionArrange the following:
CH3CH2CH2Cl (I), CH3CH2−CHCI−CH3 (II), (CH3)2CHCH2CI (III) and (CH3)3C−CI (IV) in order of decreasing tendency towards SN2 reactions
View Solution
