Which of the following molecules has longest C=C strenght?
A
CH2=C−CH2
B
CH3−CH=CH2
C
CH3−CH3∣C∣CH3−CH=CH2
D
CH3−C∣CH3=CH2
Video Solution
Text Solution
The correct Answer is:D
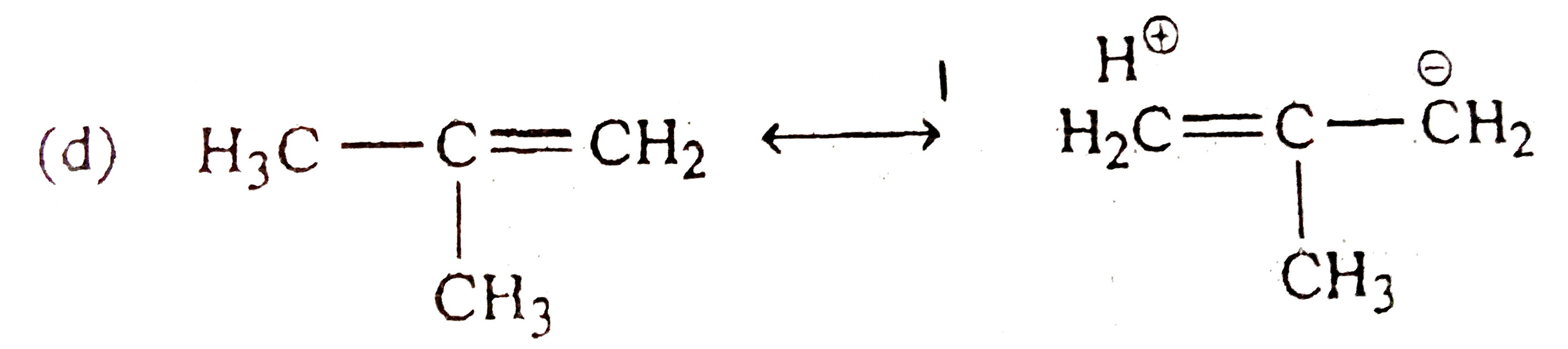
Compound contains 6α−H, so more hyperconjugation occurs.
|
Similar Questions
Which of the following has the longest C - O bond ?
View SolutionWhich of the following molecules has maximum carbon atoms in the longest chain?
View SolutionWhich molecule has the longest carbon chain?
View SolutionWhich molecule has the longest carbon chain?
View SolutionWhich of the following molecules has maximum carbon atoms in the longest chain?
View SolutionWhich molecule has the longest carbon chain?
View SolutionWhich molecule has the longest carbon chain?
View SolutionWhich of the following molecule has maximum (C−C) bond length?
View Solution
