Arrange the following compounds in decreasing order of acidity :
CH3CH2OH 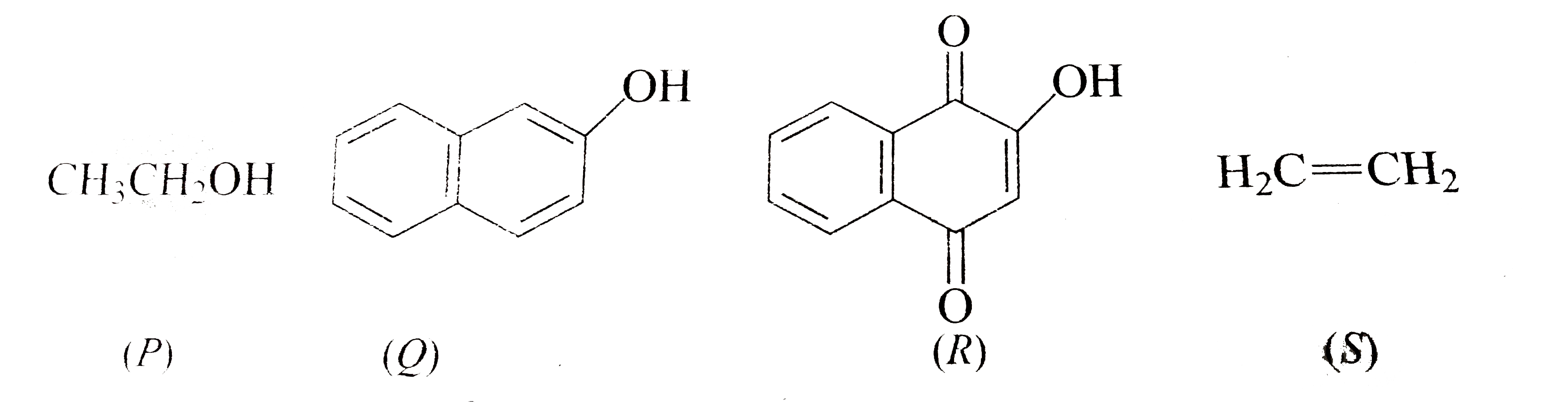
H2C=CH2
R>Q>P>S
Q>R>P>S
S>R>Q>P
Q>P>R>S
The correct Answer is:A
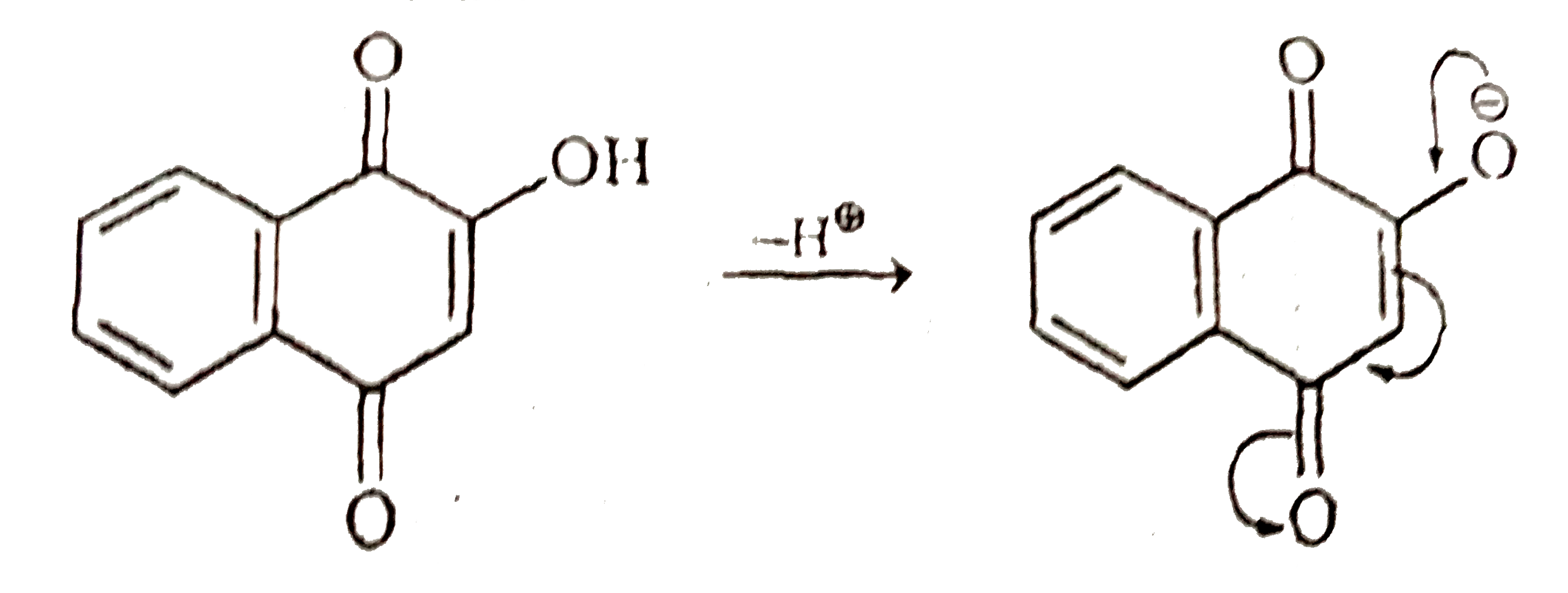
R is most acidic
Similar Questions
Knowledge Check
Arrange the following compounds in decreasing order of acidity.
(i) ClCH2CH2CH2COOH
(ii) CH3CHClCH2COOH
(iii) CH3CH2CHClCOOHAI>II>IIIBIII>II>ICI>III>IIDIII>I>II
Arrange the following compounds in the increasing order of their boiling points:
CH3CH2CH2CHO,CH3CH2CH2CH2OH,H5C2−O−C2H5,CH3CH2CH2CH3
View SolutionArraange the following compounds in increasing order of dipole moment?
CH3CH2CH3.CH3CH2NH2,CH3CH2OH
View SolutionArrange the following compounds in increasing order of their boiling points.
CH3CHO,CH3CH2OH,CH3OCH3,CH3CH2CH3
View SolutionArrange the following compounds in increasing order of their boiling points :
CH3CHO,CH3CH2OH,CH3OCH3,CH3CH2CH3
View SolutionArrange the following compounds in the increasing order of their boiling points:
CH3CH2CH2CHO,CH3CH2CH2CH2OH,H5C2−O−C2H5,CH3CH2CH2CH3
View SolutionArrange the following compound in decreasing order of their basicity.
(a)H2C=CHNa (b)CH3CH2Na
(c)CH3CH2ONa (d)HC≡CNa
View SolutionArrange the following compounds in increasing order of their boiling points.
CH3CHO,CH3CH2OH,CH3OCH3,CH3CH2CH3
View Solution

